Atom Drawing Of Hydrogen
Atom Drawing Of Hydrogen - Niels bohr, danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. Yet, shifting society toward hydrogen energy requires overcoming some significant technical issues. However, it's easy to determine the configuration of electrons for heavier elements by making a chart. Web to write the orbital diagram for the hydrogen (h) first we need to write the electron configuration for just h. An electron, bearing one unit of negative electrical charge, is also associated with this nucleus. Distinguish between the bohr and schrödinger models of the atom. Web bohr’s theory explained the atomic spectrum of hydrogen and established new and broadly applicable principles in quantum mechanics. These images show (a) hydrogen gas, which is atomized to hydrogen atoms in the discharge tube; Web the hydrogen atom has a nucleus consisting of a proton bearing one unit of positive electrical charge; Hydrogen greg robson/cc by 2.0 helium greg robson/cc by 2.0 lithium greg robson/cc by 2.0 lithium is the first element in which an additional. Web the next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell. Specifically it demonstrated that the integers in the rydberg formula are a manifestation of quantization. Web hydrogen was recognized as a distinct substance by henry cavendish in 1776. Hydrogen and helium the most common element in. Diagram of a simple hydrogen atom. Use quantum numbers to calculate important information about the hydrogen atom. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. Web a hydrogen atom is an atom of the chemical element hydrogen. If they didn't exist, the boiling point of. That’s not including “dark matter,” which is beyond the scope of this. Bohr's model does not work for systems with more than one electron. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. An electron, bearing one unit of negative electrical charge, is also associated. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula h2. Web the next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell. The heavier elements were originally made from hydrogen atoms or from other elements that were originally made. The hydrogen atom consists of an electron and a proton bound together by the attractive electrostatic force between the negative and positive charges of these particles. Hydrogen is the most abundant of all elements in the universe. Web hydrogen is a chemical element; Hydrogen forms weak bonds between molecules, latching onto adjacent oxygen, nitrogen or fluorine atoms. Web to draw. Bohr’s proposal explained the hydrogen atom spectrum, the origin of the rydberg formula, and the value of the rydberg constant. It has symbol h and atomic number 1. Specifically it demonstrated that the integers in the rydberg formula are a manifestation of quantization. H ν = δ e = ( 1 n l o w 2 − 1 n h. Diagram of a simple hydrogen atom. If they didn't exist, the boiling point of. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. Thus the most probable radius obtained from quantum mechanics is identical to the radius calculated by classical mechanics. Niels bohr, danish physicist, used the planetary. Bohr's model does not work for systems with more than one electron. Web bohr’s theory explained the atomic spectrum of hydrogen and established new and broadly applicable principles in quantum mechanics. Niels bohr, danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. Web hydrogen was recognized as a distinct. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. Web the hydrogen atom has a nucleus consisting of a proton bearing one unit of positive electrical charge; It has symbol h and atomic number 1. Distinguish between the bohr and schrödinger models of the atom. H ν = δ e = ( 1 n l o w. Use quantum numbers to calculate important information about the hydrogen atom. Niels bohr, danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. Specifically it demonstrated that the integers in the rydberg formula are a manifestation of quantization. Hydrogen forms weak bonds between molecules, latching onto adjacent oxygen, nitrogen or. Web the next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell. Specifically it demonstrated that the integers in the rydberg formula are a manifestation of quantization. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. Niels bohr, danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. Web historically, bohr’s model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. Niels bohr, danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. However, it's easy to determine the configuration of electrons for heavier elements by making a chart. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. Bohr’s proposal explained the hydrogen atom spectrum, the origin of the rydberg formula, and the value of the rydberg constant. If they didn't exist, the boiling point of. Hydrogen greg robson/cc by 2.0 helium greg robson/cc by 2.0 lithium greg robson/cc by 2.0 lithium is the first element in which an additional. It's these hydrogen bonds that give water many of its properties. Web elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the coulomb force. Distinguish between the bohr and schrödinger models of the atom.
Describe Bohr’s model of the hydrogen atom. bitWise Academy
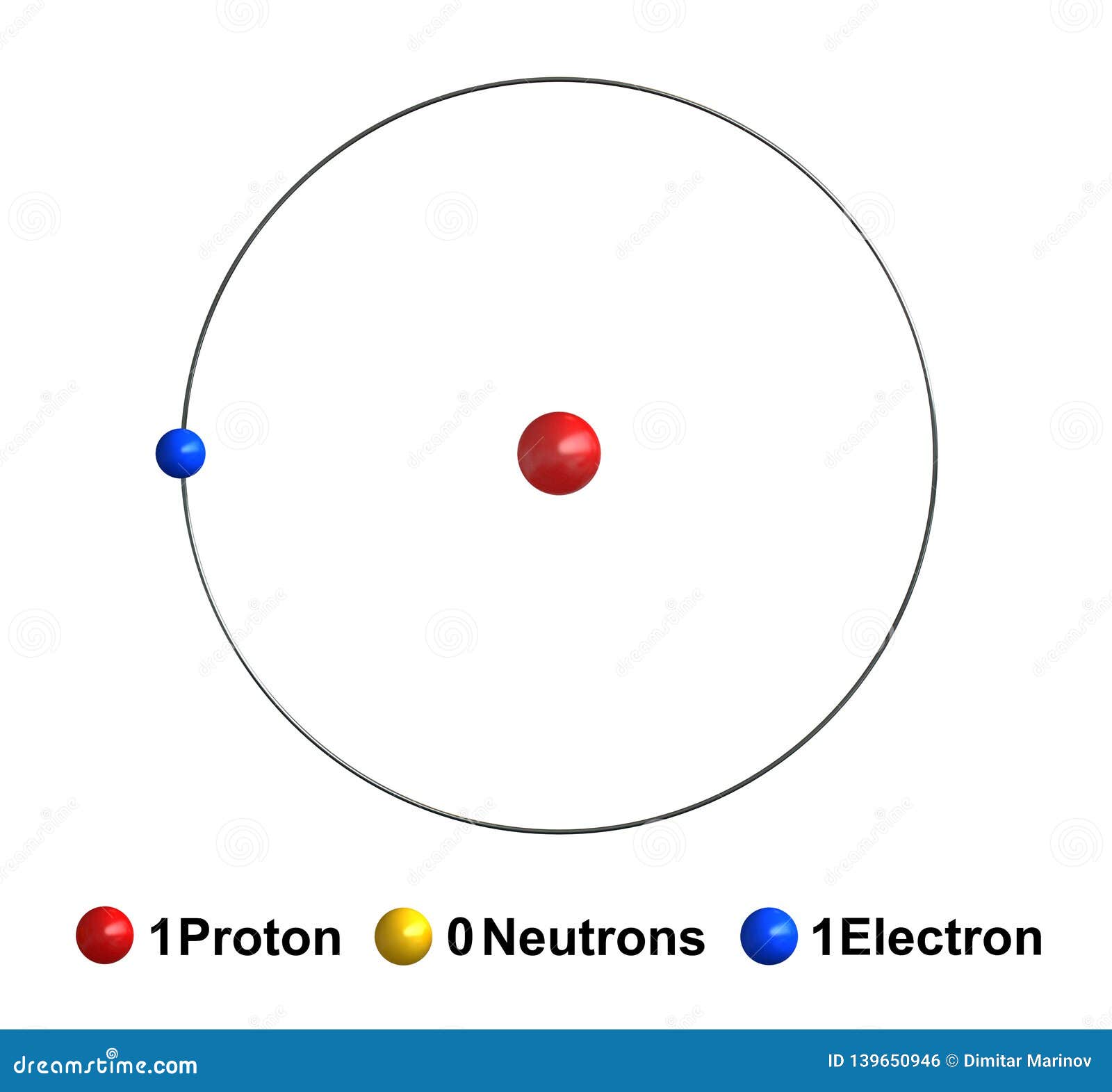
Hydrogen stock illustration. Illustration of education 139650946

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
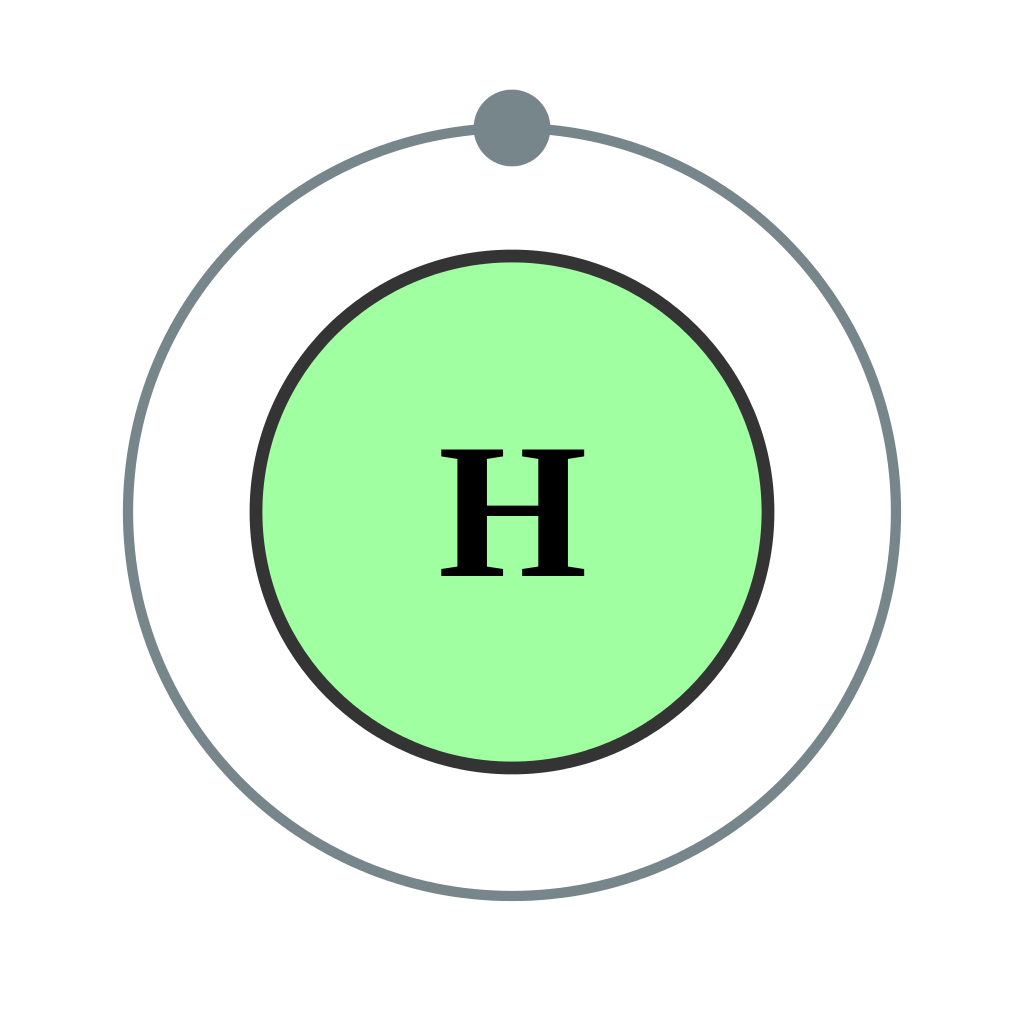
FileElectron shell 001 Hydrogen no label.svg Wikipedia

Diagram representation element hydrogen Royalty Free Vector

Hydrogen Definition, Structure, Properties & Uses Embibe

Hydrogen atom on white background Royalty Free Vector Image

Diagram Representation Of The Element Hydrogen Stock Vector Image

Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy
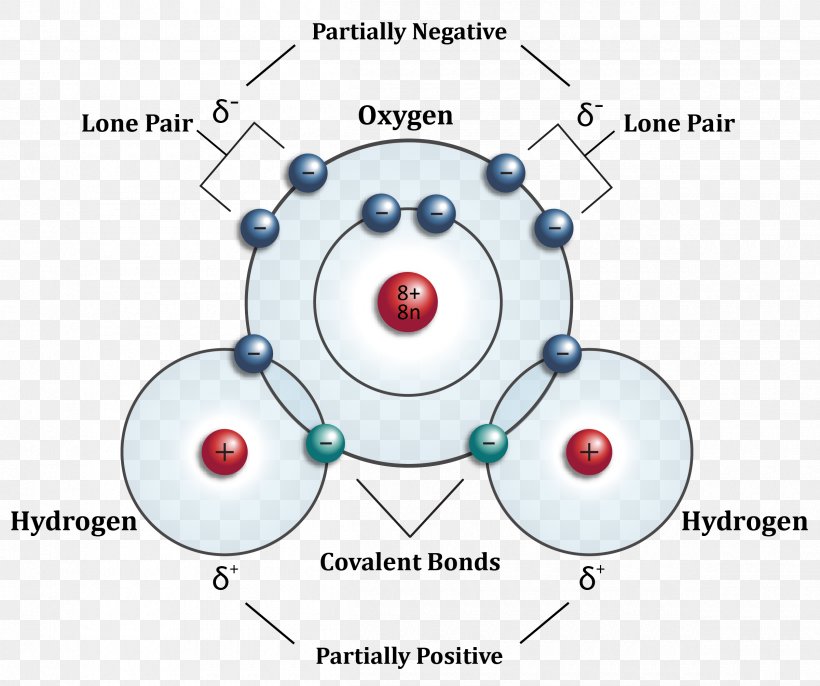
Hydrogen Molecule Structure
Therefore, It Is Important To Further Study The Grating Formation Mechanism Of Fiber Gratings For The Rapid.
Under Ordinary Conditions, Hydrogen Gas Is A Loose Aggregation Of Hydrogen Molecules, Each Consisting Of A Pair Of Atoms, A Diatomic.
Web To Write The Orbital Diagram For The Hydrogen (H) First We Need To Write The Electron Configuration For Just H.
Hydrogen And Helium The Most Common Element In The Universe Is Hydrogen, A Gas That Makes Up About 99% Of The Universe’s Known Mass.
Related Post: