Atom Of Carbon Drawing
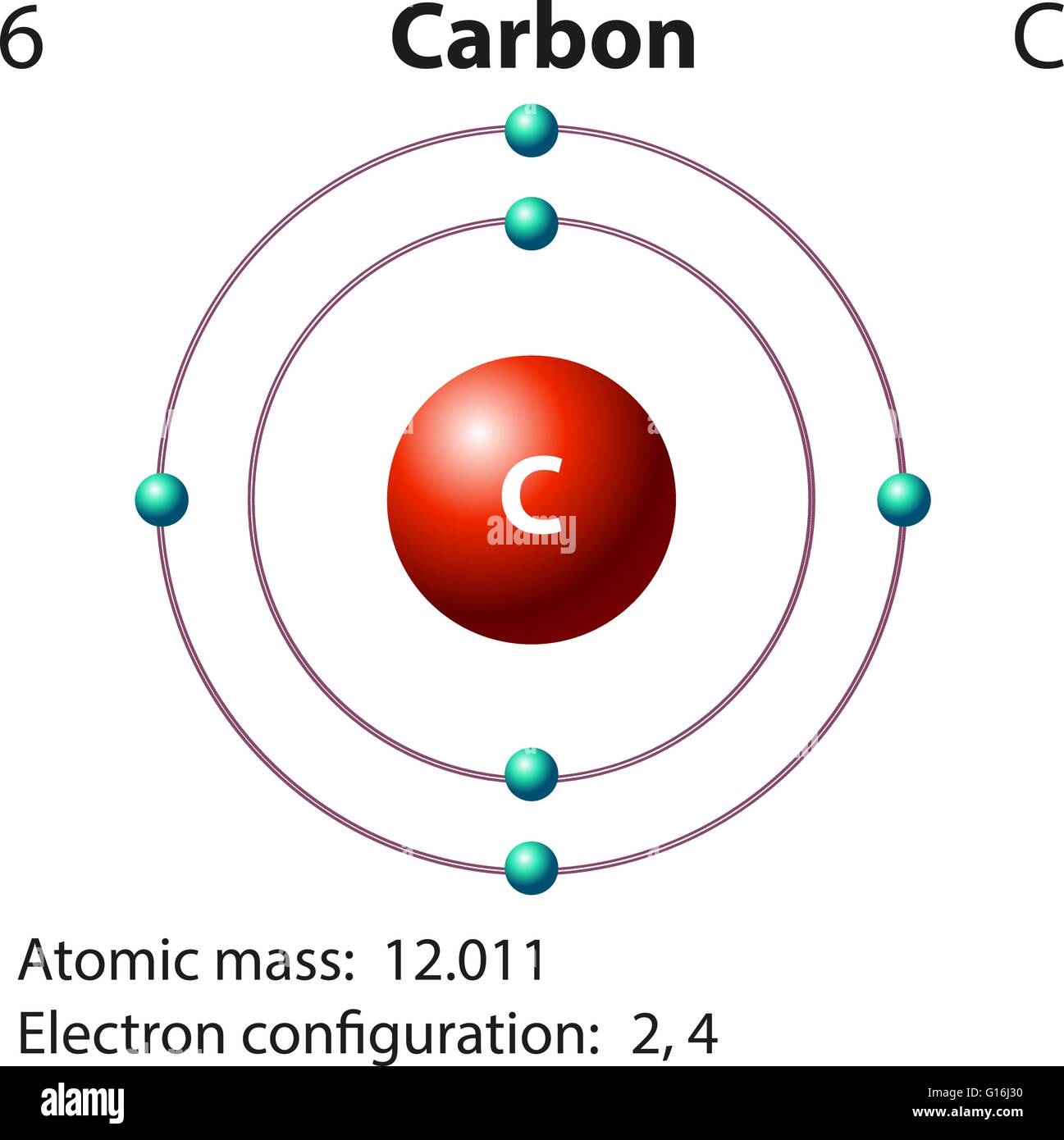
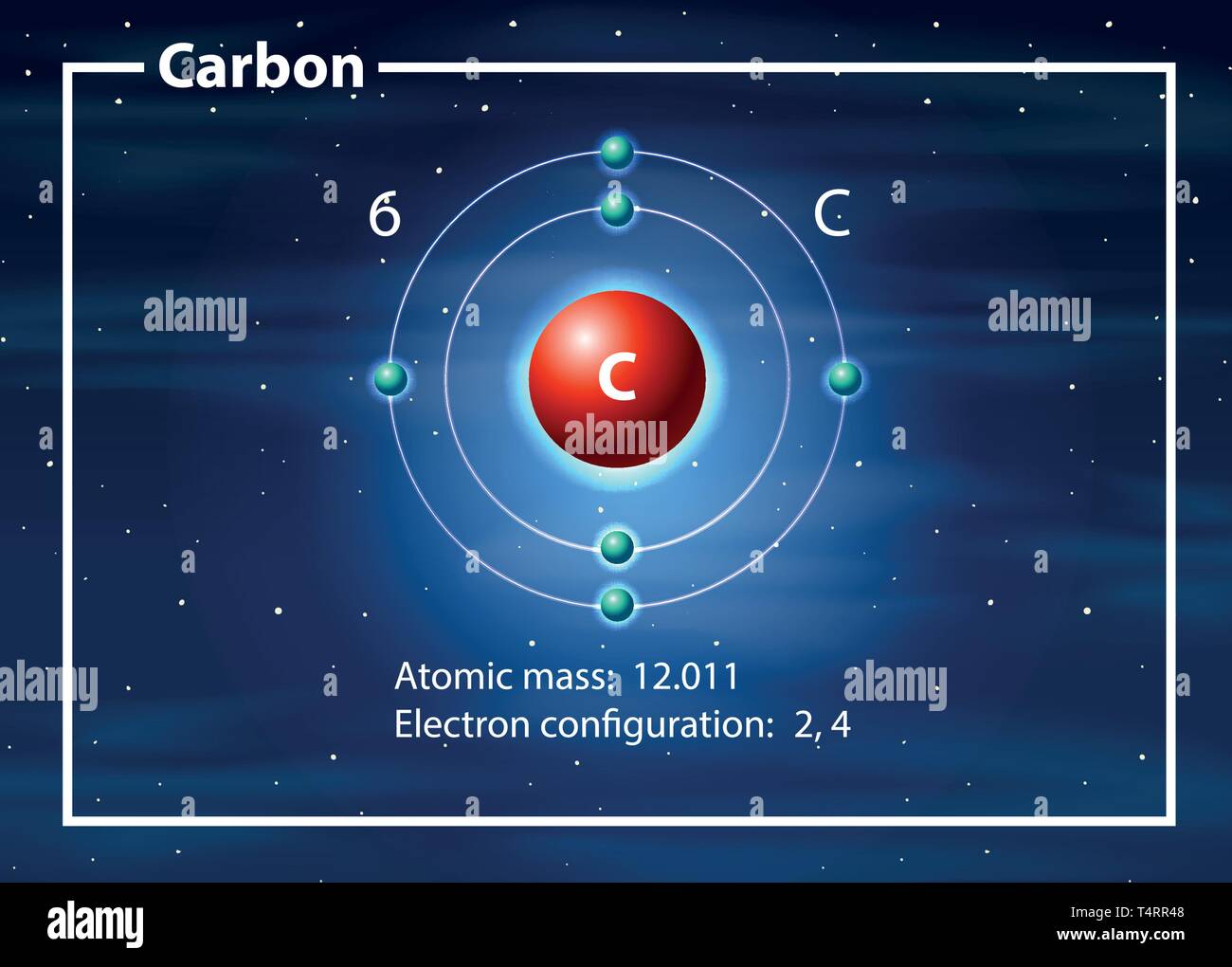
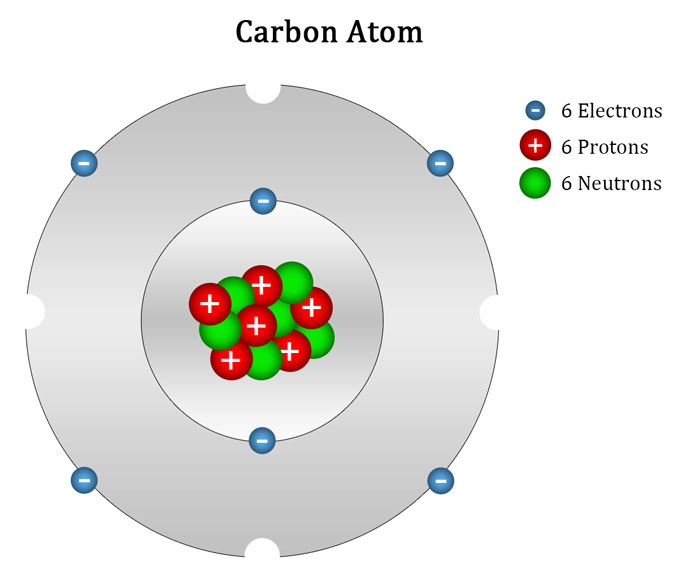
Atom Of Carbon Drawing - Web here's hydrogen, in group one, so one valence electron. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Web step 1: Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Carbon atoms aren’t usually shown. Rule 1 carbon atoms aren’t usually shown. It makes the geometrical structure of the carbon monoxide linear. Hybridization can be understood by 2 methods, one by understanding the combination of the orbitals and 2nd by using a simple formula. Lewis dot diagram of carbon. In the bohr model, electrons are pictured as traveling in circles at different shells,. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds. Web bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Web for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Web a carbon atom is present wherever a line intersects another line. Carbon (atomic number. Web the hybridization of co2 is sp. Web the rules for drawing skeletal structures are straightforward. Web the rules for drawing skeletal structures are straightforward. Rule 2 hydrogen atoms bonded to carbon aren’t shown. Both the atoms will be central; The number of protons and the mass number of an atom define the type of atom. Rule 2 hydrogen atoms bonded to carbon aren’t shown. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. Add enough electrons (dots) to the outer atoms to. It. Web the rules for drawing skeletal structures are straightforward. The carbon atom is sp hybridized and oxygen atoms are sp2, making the overall molecule sp hybridized. There are enough hydrogen atoms attached to each carbon to make the total number of bonds on that carbon up to 4. So let's go back down and let's calculate the total number of. Carbon has 2 electrons in its first shell and 4 in its second shell. 13k views 11 years ago. Geometrical shape of the carbon monoxide (co) the bond angle between the carbon and the oxygen atom is 180 degrees. Lone pair electrons are usually omitted. Hydrogen atoms are omitted but are assumed to be present to complete each of carbon's. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Carbon (atomic number 6) has six electrons. Rule 1 carbon atoms aren’t usually shown. Occasionally, a carbon atom might be indicated for emphasis or clarity. It is nonmetallic and tetravalent—meaning that its atoms are able to form up. Web here's hydrogen, in group one, so one valence electron. The number of protons and the mass number of an atom define the type of atom. Web bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Web carbon (from latin carbo 'coal') is a chemical element; Instead, a carbon atom is assumed. Carbon atoms aren’t usually shown. Therefore, a carbon atom will have two electrons in the first shell and four in the 2nd shell. Occasionally, a carbon atom might be indicated for emphasis or clarity. Web the atomic number of carbon is 6. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that. 13k views 11 years ago. Web step 1: Lone pair electrons are usually omitted. The number of protons and the mass number of an atom define the type of atom. Therefore, a carbon atom will have two electrons in the first shell and four in the 2nd shell. Web the rules for drawing skeletal structures are straightforward. Web drawing a carbon atom. 1 without carbon, you wouldn't have the plasma membranes of your cells, the sugar molecules you use for fuel, or even the dna that carries instructions to build and run your body. So let's go back down and let's calculate the total number of valence electrons that we need to represent in our dot structure. Web carbon (from latin carbo 'coal') is a chemical element; Web the hybridization of co2 is sp. 13k views 11 years ago. Video showing how to draw a carbon atom.more. Web the atomic number of carbon is 6. Hydrogens that are attached to elements other than carbon are shown. Web about 18% of your body consists of carbon atoms, by mass, and those carbon atoms are pretty key to your existence! It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Web the bohr model of carbon is drawn with only two electron shells, the first shell contains 2. Both the atoms will be central; The number of protons and the mass number of an atom define the type of atom.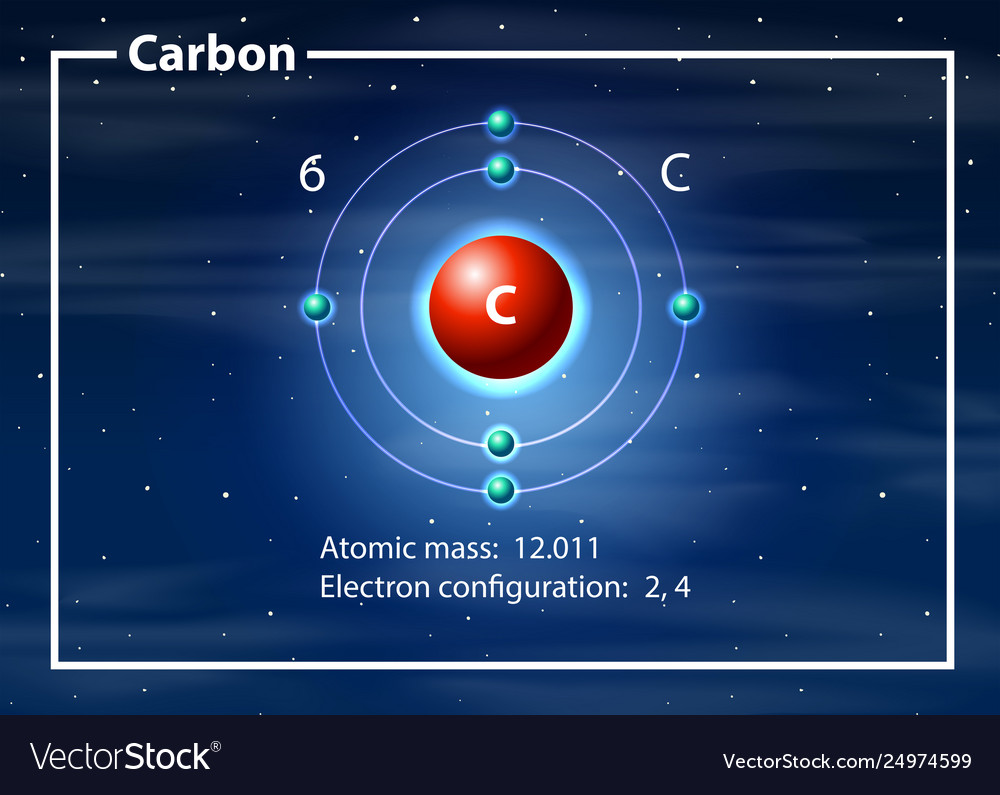
Carbon atom diagram concept Royalty Free Vector Image
Carbon atom diagram hires stock photography and images Alamy
Carbon atom diagram hires stock photography and images Alamy
Carbon Atom Ascension Glossary
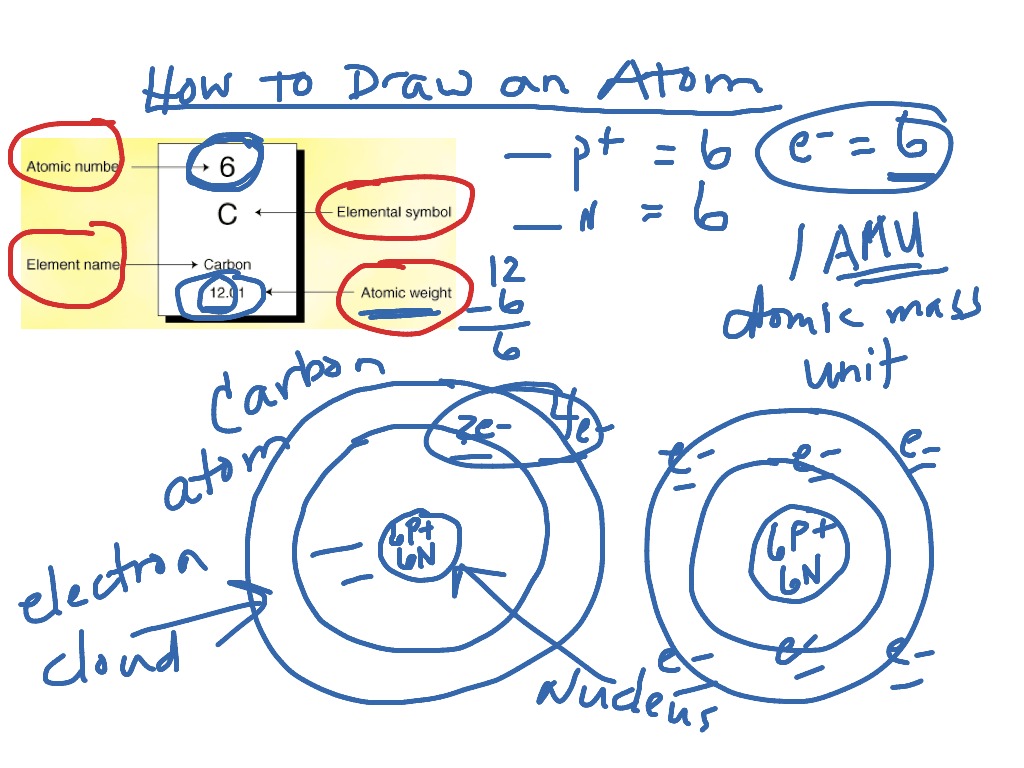
How to draw an atom of carbon Science ShowMe
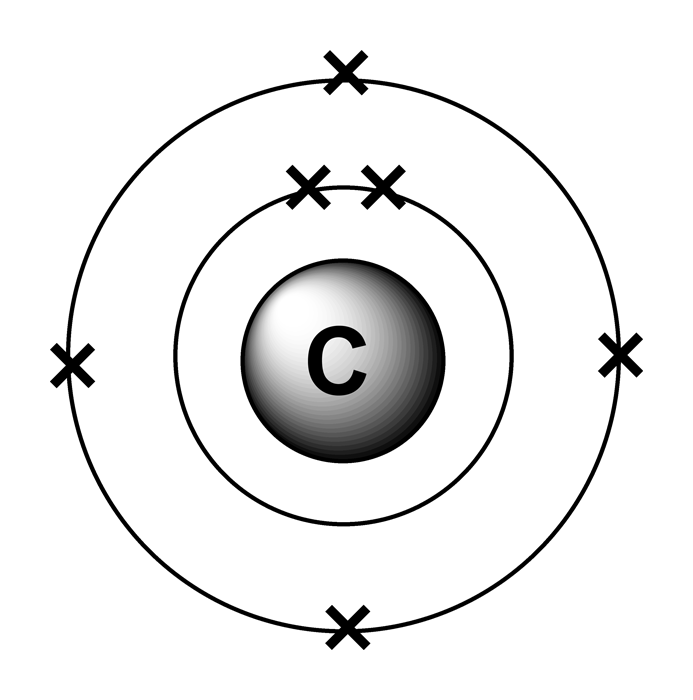
Electron configurations

Carbon atomic structure (437243) Illustrations Design Bundles
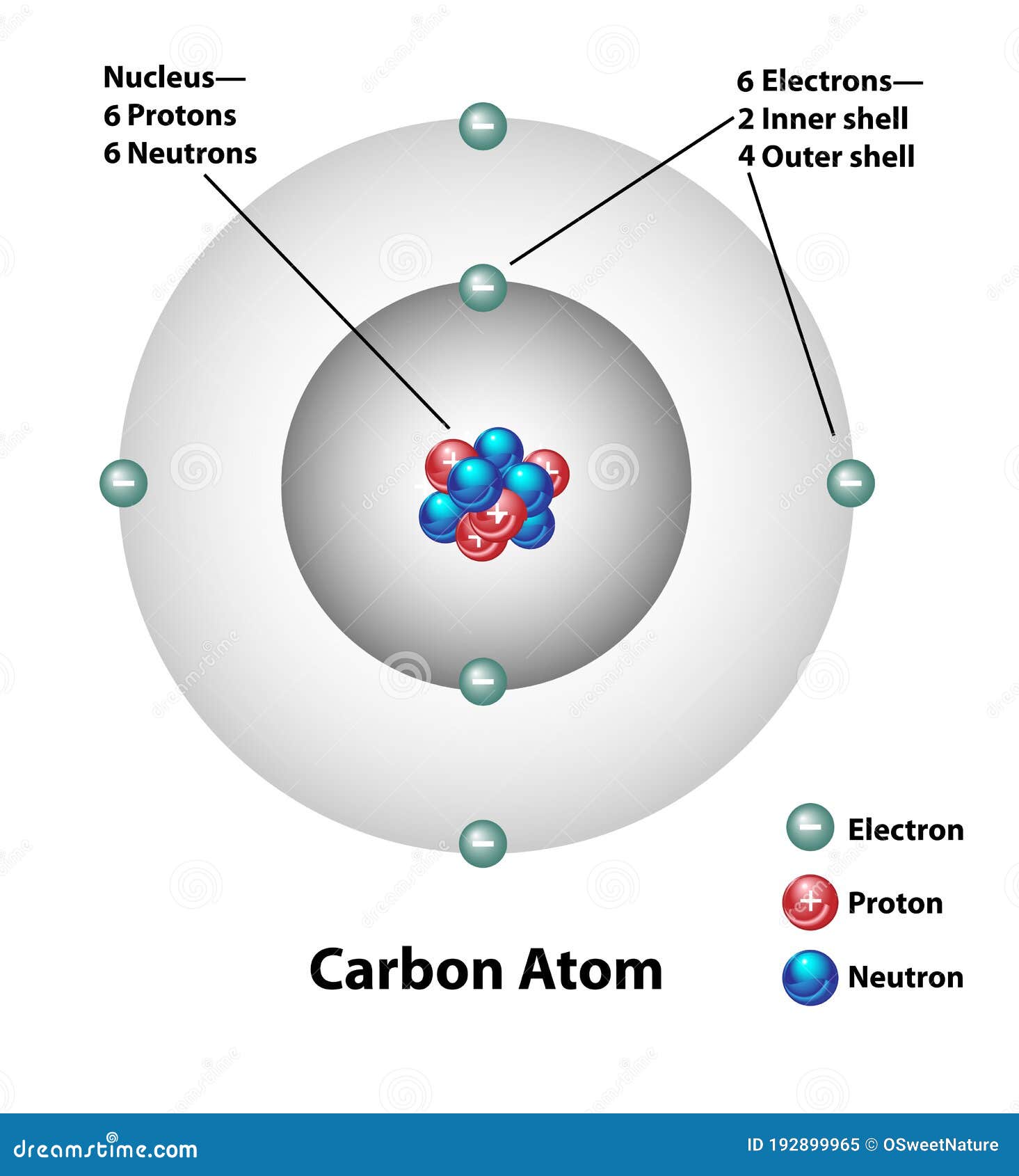
Carbon Atom Molecular Structure Labels Stock Vector Illustration of

Download Carbon Atom Atoms RoyaltyFree Stock Illustration Image Pixabay

Carbon atoms hires stock photography and images Alamy
In The Bohr Model, Electrons Are Pictured As Traveling In Circles At Different Shells,.
Web For Example, Each Atom Of A Group 14 Element Has Four Electrons In Its Outermost Shell And Therefore Requires Four More Electrons To Reach An Octet.
Rule 2 Hydrogen Atoms Bonded To Carbon Aren’t Shown.
Lewis Dot Diagram Of Carbon.
Related Post: