Draw A Lewis Structure For Hcn
Draw A Lewis Structure For Hcn - Web 6 steps to draw the lewis structure of hcn step #1: Here we have to find the valence electrons of all three atoms, hydrogen, carbon, and nitrogen. Web drawing the lewis structure for hcn. The sum of the valence electrons is 5 (from n) + 6 (from o) = 11. You'll get a detailed solution from a subject matter expert. This problem has been solved! Draw a skeleton structure put the least electronegative atom c in the middle with h and cl on either side. Chemistry 1 answer ernest z. 📌you can draw any lewis structures by following the simple steps mentioned.more Draw lewis structures depicting the bonding in simple molecules. The foremost step of creating a lewis structure is finding the valence electrons. Step method to draw the lewis structure of hcn. We'll also compare hnc to hcn and discuss why both are of inter. Here is a little flow chart of how we are going to do this: Web the first step is to sketch the lewis structure of. Determine the total number of valence (outer shell) electrons. Chemistry 1 answer ernest z. At least two lewis structures can be drawn for bcl 3. Web 6 steps to draw the lewis structure of hcn step #1: Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. The second step is to add valence electrons to the one hydrogen atom, and the final step is to combine the. However, there are two possible resonance structures that can be drawn for hcn: Counting valence electrons before we can start drawing the lewis structure, we need to determine the total. Web here are the following steps needed to draw. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the lewis structure of hcn shows a triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. The second step is to add valence electrons to the one hydrogen atom, and the final step is. Draw at least one other lewis structure for the nitrate ion that is not plausible based on formal charges. Determining the formal charge formal charge is a vital concept in drawing lewis structures. Nov 12, 2017 here's how to do it. This molecule is made up of three different atoms: In this example problem, we draw the lewis structure. Here is a little flow chart of how we are going to do this: In order to draw the lewis. Using arguments based on formal charges, explain why the. Select the center atom (h is always outside). Calculate the total number of valence electrons. Web learn the steps to draw the lewis structure of hcn in just 1 minute. Draw lewis structures depicting the bonding in simple molecules. Draw at least one other lewis structure for the nitrate ion that is not plausible based on formal charges. Calculate the total number of valence electrons. Enjoy 2 weeks of live tv, on us stream more,. Does this molecule exhibit resonance? Web the lewis structure (lewis dot diagram) for hcn.1. In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you. Web here are the following steps needed to draw the lewis structure of hcn: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web how to draw lewis structure for hcn? Calculate the total number of valence electrons. Using arguments based on formal charges, explain why the. In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Web chemistry chemistry questions and answers draw the lewis structure hcn and then determine its electron. Web drawing the lewis structure for hcn. While selecting the atom, always put the least electronegative. Web science chemistry chemistry questions and answers draw the lewis structure for hcn. Draw the lewis structure for hcn. Calculate the total number of valence electrons. To know the valence electrons of hcn, let us go through the valence electrons of individual atoms in hydrogen cyanide. Put one electron pair in each bond4. 📌you can draw any lewis structures by following the simple steps mentioned.more In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Step method to draw the lewis structure of hcn. Web 6 steps to draw the lewis structure of hcn step #1: After determining how many valence electrons there are. Web the lewis structure (lewis dot diagram) for hcn.1. Nov 12, 2017 here's how to do it. Web learn the steps to draw the lewis structure of hcn in just 1 minute.
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
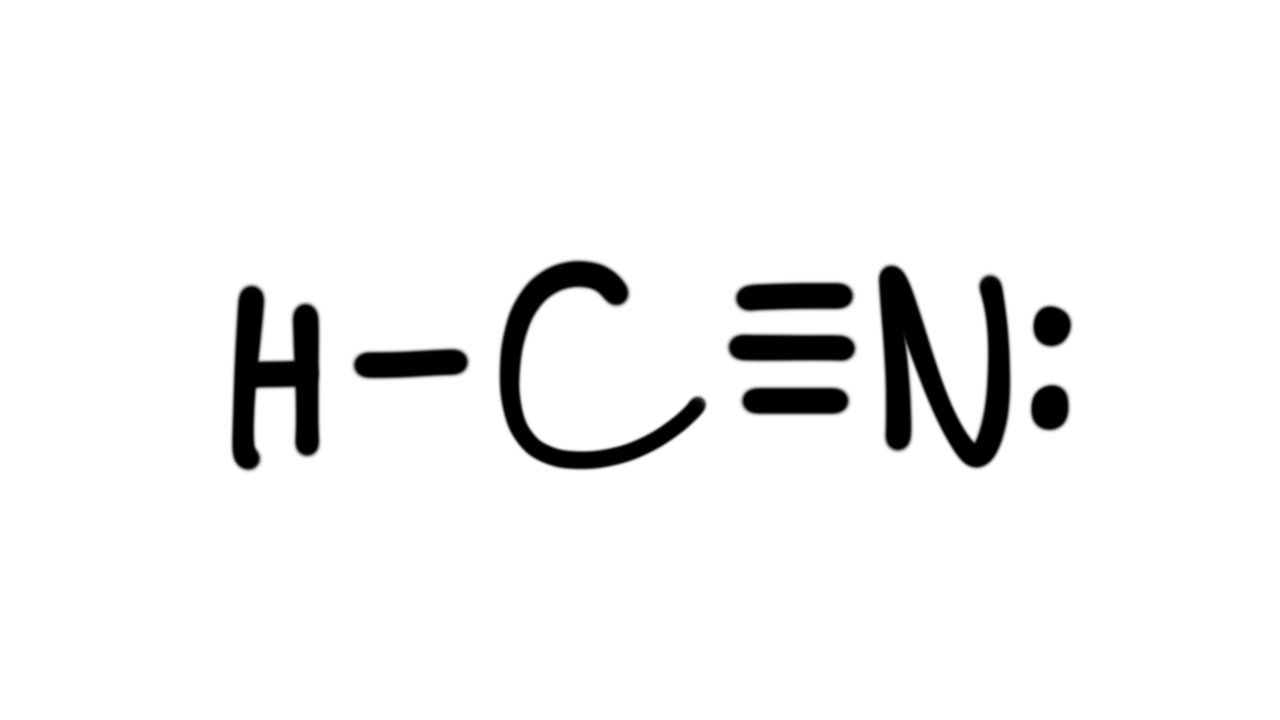
Lewis Diagram For Hcn

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw

How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN

Hcn Lewis Structure Bonds Draw Easy
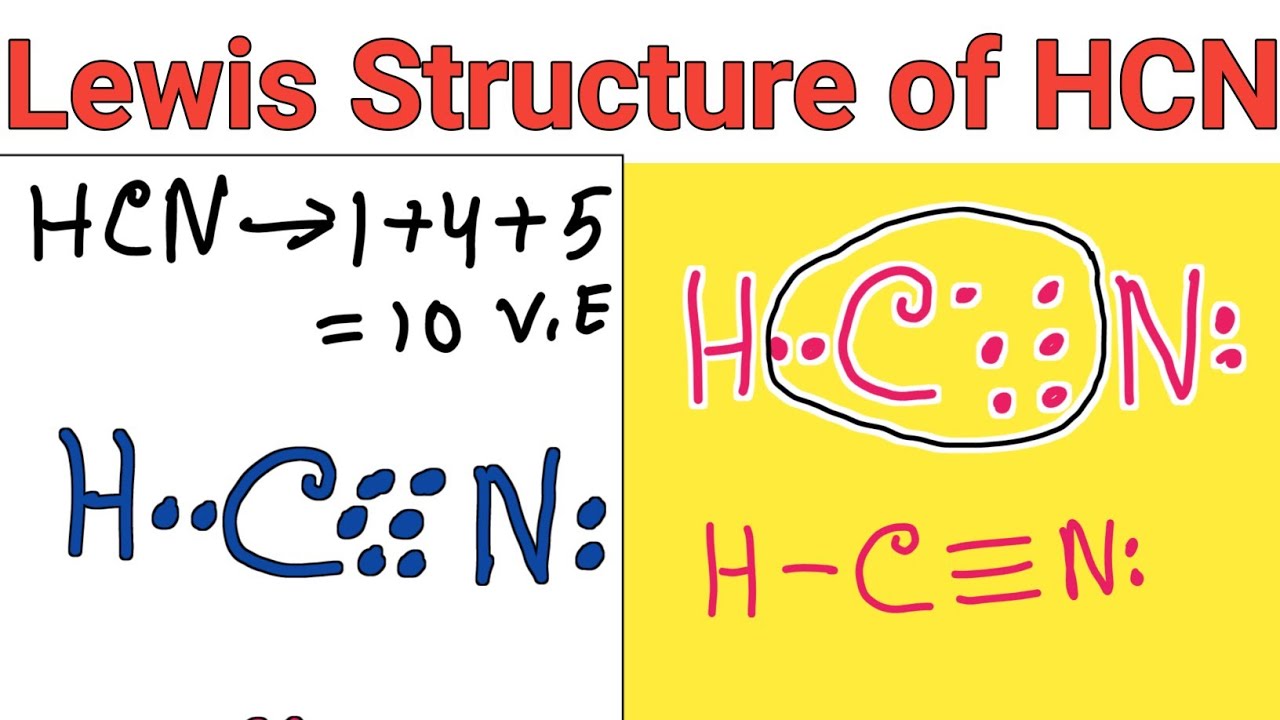
Lewis structure of HCN (Hydrogen cyanide) YouTube
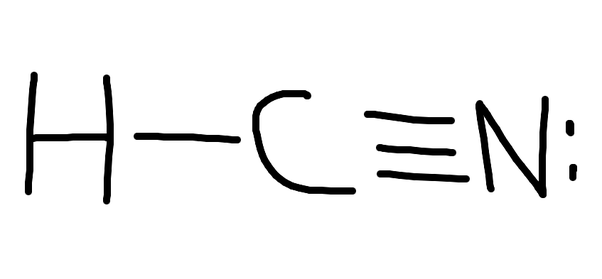
Lewis Diagram For Hcn

Lewis Dot Diagram Of Hcn
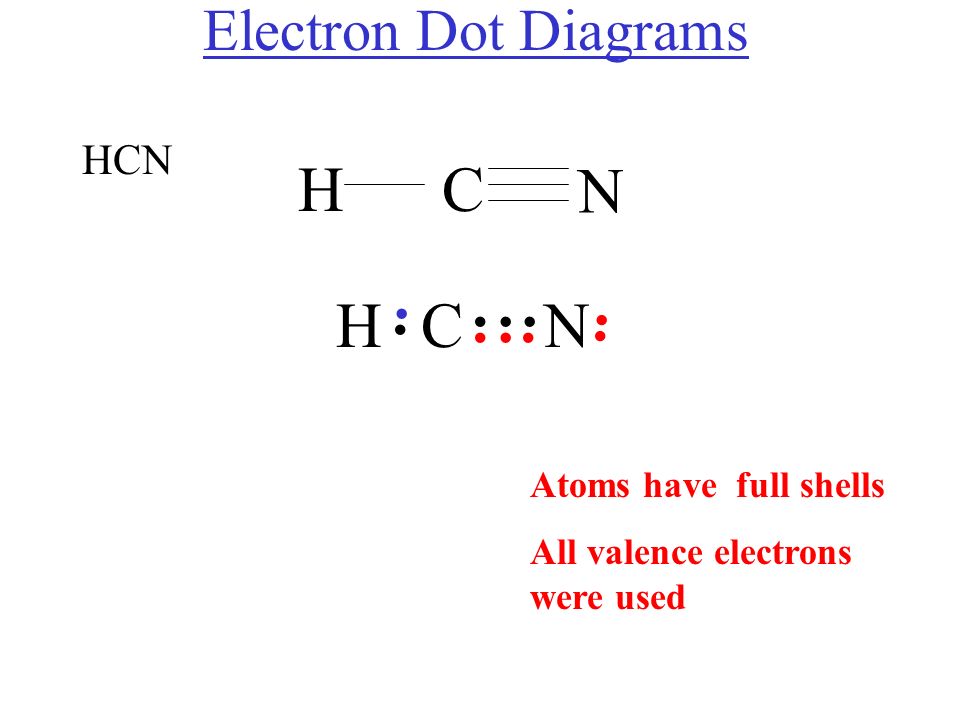
Lewis Diagram For Hcn

HCN Lewis Structure (Hydrogen Cyanide) Molecules, Chemical formula, Lewis
Put Least Electronegative Atom In Centre3.
Web Chemistry Chemistry Questions And Answers Draw The Lewis Structure Hcn And Then Determine Its Electron Domain And Molecular Geometries This Problem Has Been Solved!
The Second Step Is To Add Valence Electrons To The One Hydrogen Atom, And The Final Step Is To Combine The.
Determining The Formal Charge Formal Charge Is A Vital Concept In Drawing Lewis Structures.
Related Post: