Draw A Lewis Structure For Nh3
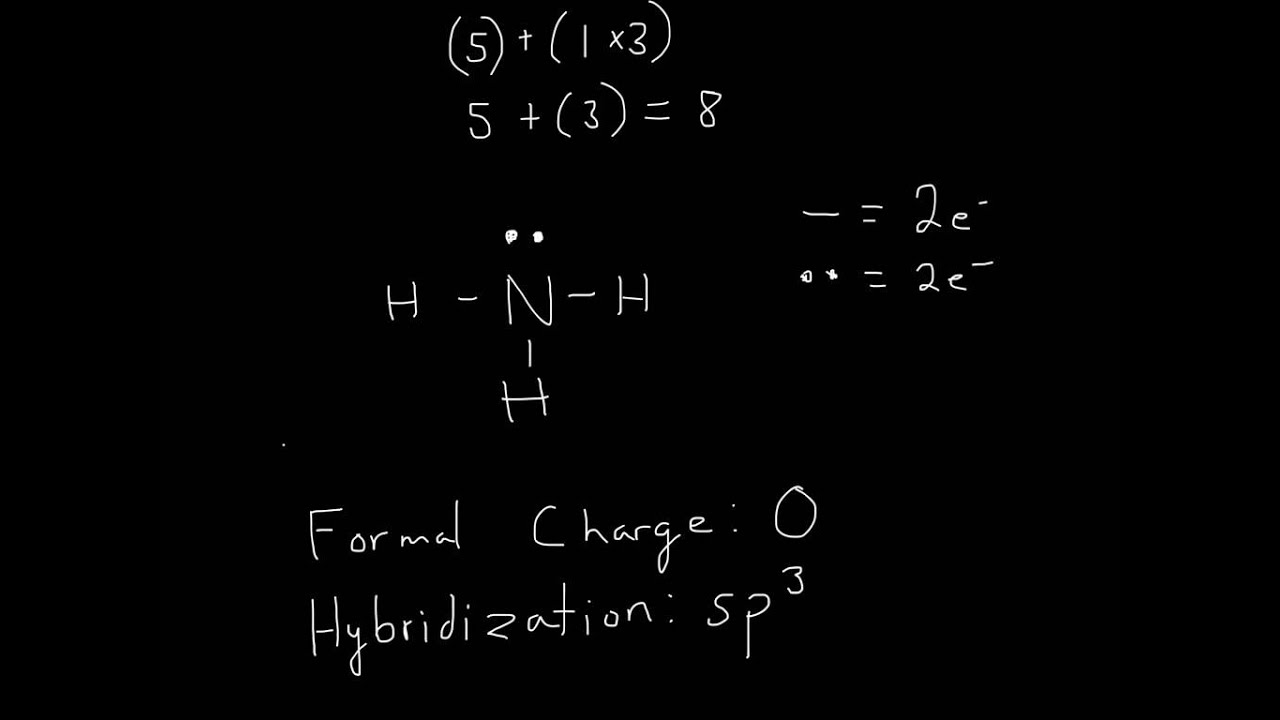
Draw A Lewis Structure For Nh3 - Determine the total number of valence electrons for all the atoms in nh3, which is 5 (for nitrogen) + (3 x 1) (for hydrogen) = 8. Count the total number of valence electrons to begin drawing the nh3 lewis structure, start by counting the total. Web steps of drawing lewis structure of nh 3 find total number of electrons of the valance shells of hydrogen atoms and nitrogen atom total electrons pairs as lone pairs and bonds center atom selection mark lone pairs on atoms mark charges on atoms if there are charges on atoms. While selecting the center atom, always put the least. Web steps of drawing nh3 lewis structure step 1: Web the first step is to sketch the lewis structure of the nh3 molecule, to add valence electrons around the nitrogen atom; It is eight to form a single nh3 molecule. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Draw the lewis structure for nh3. It has one valence electron, but we. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer. It has one valence electron, but we. Drawing the lewis structure for nh3 it is helpful if you: If the species is an ion, add or subtract electrons corresponding to the charge of the ion. A) draw the lewis structure. Valence electrons are the electrons in the outermost energy level of an atom. Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Web 0:00 / 3:17 lewis structure: Determine the central atom in the nh3 molecule, nitrogen (n) is the central atom since it is less electronegative. Web the first step is. Place the nitrogen atom in the center of the structure and. Calculate the total number of valence electrons. Web we're going to do the lewis structure for nh3: It is six for one ammonia (nh3) molecule according to the octet rule. It also is a good example of a molecule with a trigonal prymidal molecular geometry. It is six for one ammonia (nh3) molecule according to the octet rule. Please include all nonbonding electrons. Calculate the total number of valence electrons. Web chemistry chemistry questions and answers draw the lewis structure of ammonia (nh3). This is the reason why ammonia acts as a lewis base, as it can donate those electrons. On the periodic table, nitrogen is in group 5 or 15 so it has 5 valence electrons, and then hydrogen is in group 1. There are 8 valence electrons available for the lewis structure for nh 3. It is six for one ammonia (nh3) molecule according to the octet rule. Place the nitrogen atom in the center of the structure. Find more chemistry widgets in wolfram|alpha. Determine the central atom in the nh3 molecule, nitrogen (n) is the central atom since it is less electronegative. Please include all nonbonding electrons. Web follow these simple steps to draw lewis dot structures: Nh3 lewis structure the lewis structure of a molecule helps understand the electron geometry, molecular geometry, polarity, and other such. For resonance structures there must be a double or triple bond present, which is not the case with nh3. Here, the given molecule is nh3 (ammonia). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web we're going to do the lewis structure for nh3: Web to draw the nh3 lewis structure, follow. Web science chemistry chemistry questions and answers draw the lewis structure for nh3. Web steps of drawing nh3 lewis structure step 1: Web steps of drawing lewis structure of nh 3 find total number of electrons of the valance shells of hydrogen atoms and nitrogen atom total electrons pairs as lone pairs and bonds center atom selection mark lone pairs. For the nh3 structure use the periodic table to find the total number of valence electrons for the nh3. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer. Draw a lewis structure for ammonia (nh3). Web 0:00 / 3:17 lewis structure: Web get the free lewis structure finder widget. Select the center atom (h is always outside). Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to include any lone pairs.b) draw a vsepr diagram for ammonia. Web this problem has been solved! There are 8 valence electrons available for the lewis structure for nh 3. Draw the lewis structure of ammonia (nh3). Web here, we need to study how the lewis structure of the nh3 molecule is drawn: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web to draw the nh3 lewis structure, follow these steps: A) draw the lewis structure for ammonia (nh3). Nh3 lewis structure the lewis structure of a molecule helps understand the electron geometry, molecular geometry, polarity, and other such properties with ease. On the periodic table, nitrogen is in group 5 or 15 so it has 5 valence electrons, and then hydrogen is in group 1. Start by counting the total number of valence electrons in the molecule. Drawing the lewis structure for nh3 it is helpful if you: It is six for one ammonia (nh3) molecule according to the octet rule. Web we're going to do the lewis structure for nh3: Web chemistry chemistry questions and answers draw the lewis structure of ammonia (nh3). This is the reason why ammonia acts as a lewis base, as it can donate those electrons. Web science chemistry chemistry questions and answers draw the lewis structure for nh3. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. For the nh3 structure use the periodic table to find the total number of valence electrons for the nh3.
Lewis Structure NH3 plus dipoles, shape, angles and formal charge

NH3 Lewis Structure How to Draw the Dot Structure for NH3 YouTube

How to draw NH3 Lewis Structure? Science Education and Tutorials

NH3 (ammonia) Lewis dot structure YouTube

How to draw NH3 Lewis Structure? Science Education and Tutorials

NH3 Molecular Geometry Science Education and Tutorials

Lewis Dot Diagram Of Nh3

NH3 Lewis Structure , Valence Electrons ,Formal Charge,Polar or Non

Lewis Dot Diagram Of Nh3
Lewis Structure of NH3 YouTube
Web = 8 Valence Ammonia Or Nh3 Has A Total Of 8 Valence Electrons.
Draw The Atoms On Paper And Put Dots Around Them To Represent Valence Electrons Of The Atom.
It Has One Valence Electron, But We.
Determine The Central Atom In The Nh3 Molecule, Nitrogen (N) Is The Central Atom Since It Is Less Electronegative.
Related Post: