Draw A Successive Ionization Energy Diagram For Aluminum
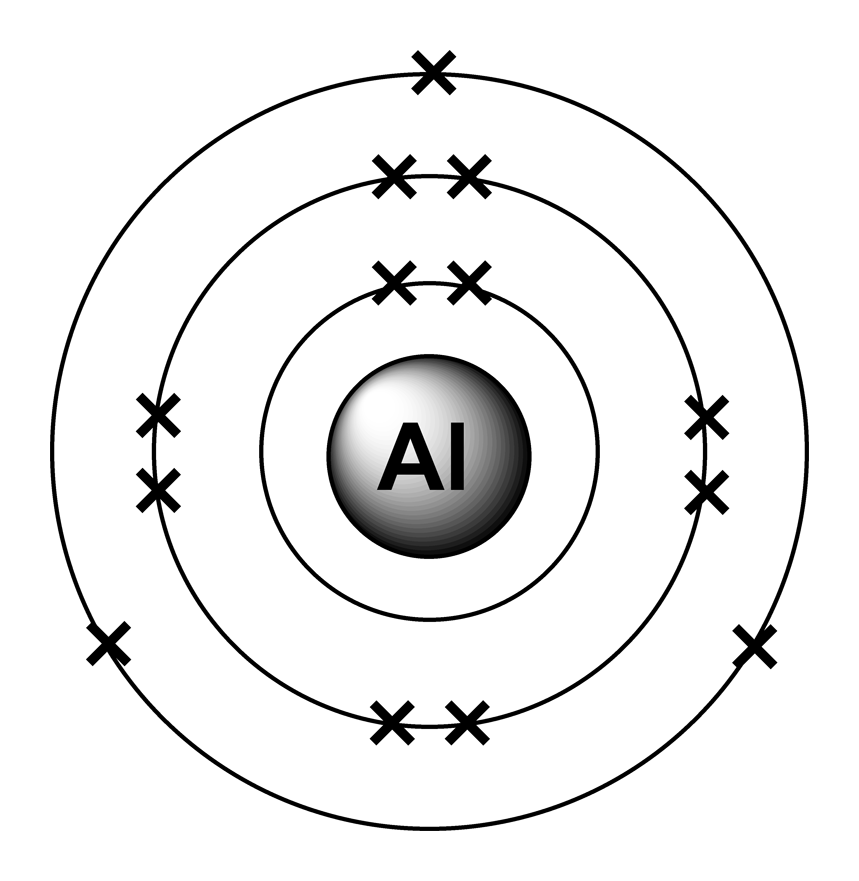
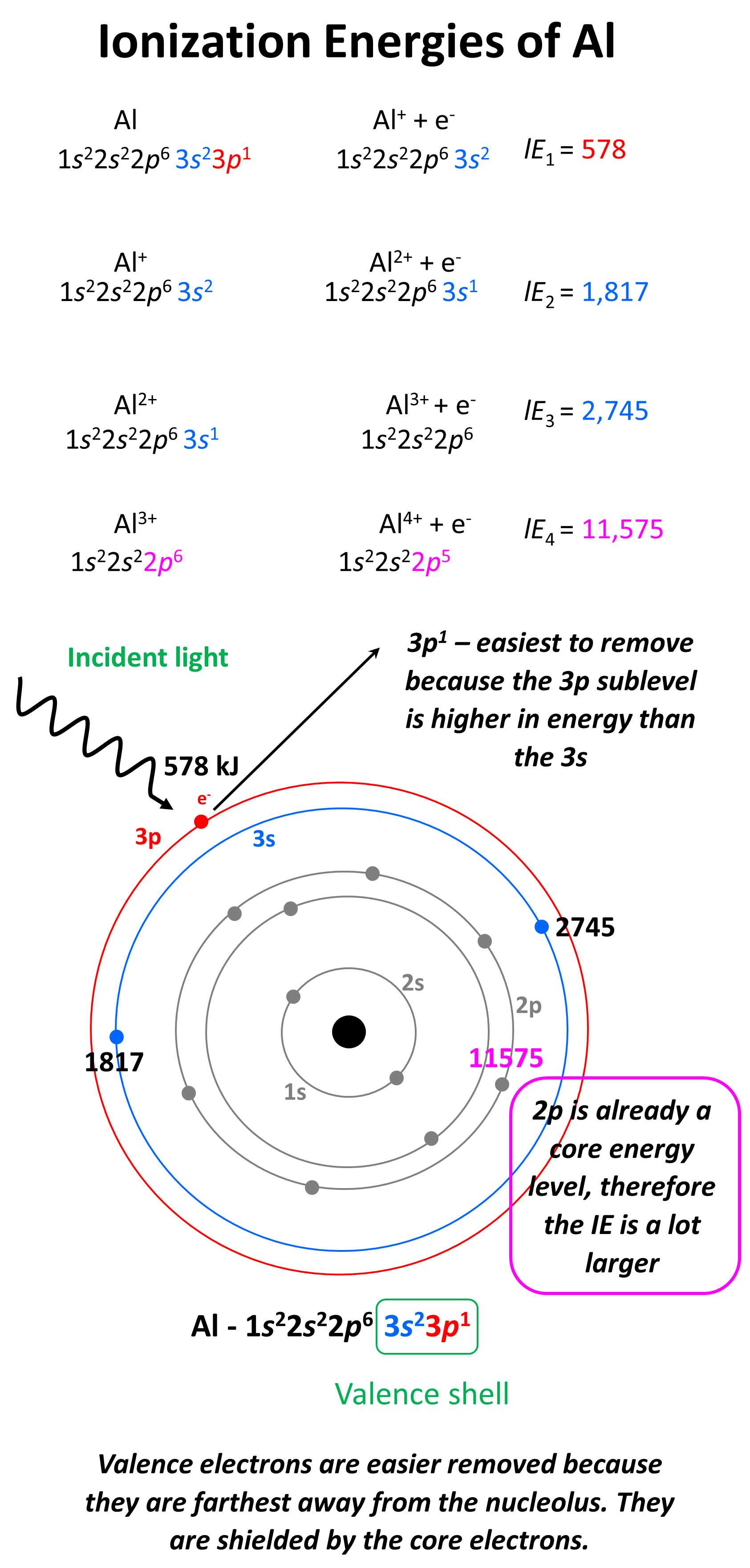
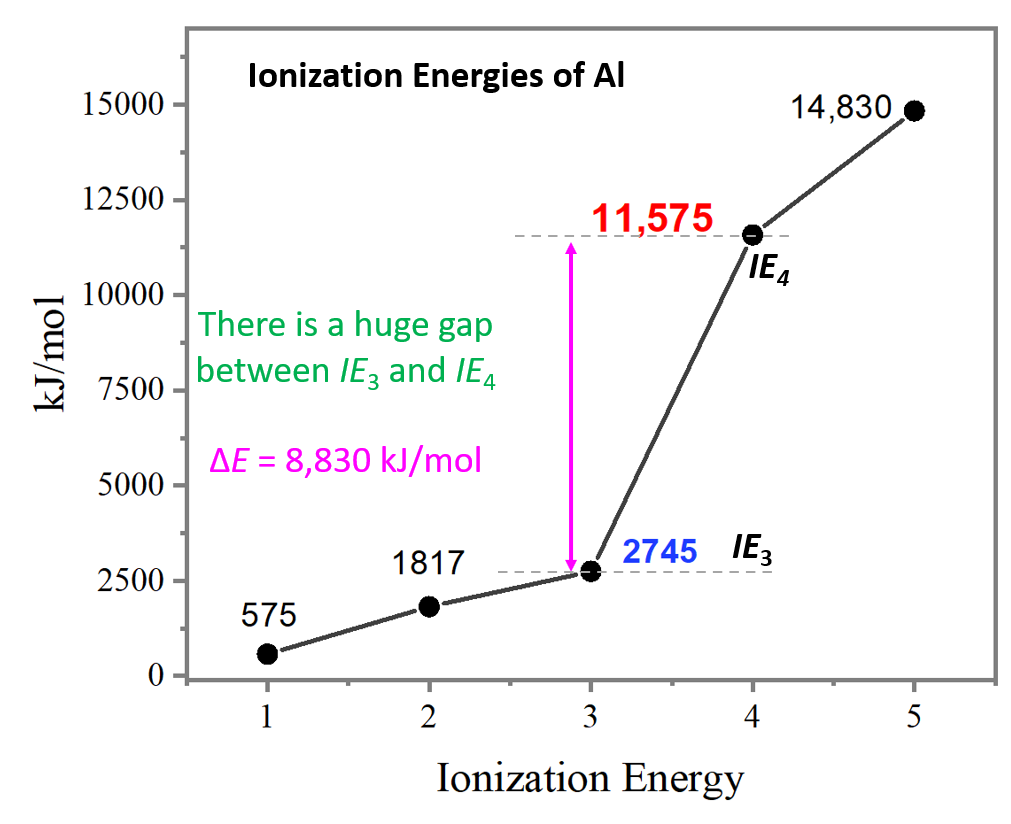
Draw A Successive Ionization Energy Diagram For Aluminum - The second ionization energy of aluminum is larger than the first, and the third ionization energy is even larger. Web in a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make covalent or ionic bonds with each other. The ionization energy is measured in joules (j) or electron volts (ev). Some of these electrons are more tightly bound in the atom than others. For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). I 2 i_2 i 2 = 1,817 kj/mol. That is because aluminum has three valence electrons that are located in the outermost shell. Web so without actually providing the ionization energies for all the group 13 elements, they could say that the element has the second highest first ionization energy in its group, which would be aluminum. Web so, this is high, high ionization energy, and that's the general trend across the periodic table. 2nd ionization energy, 1816 kj ⋅ mol−1; Periodic properties of the elements For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). I 3 i_3 i 3 = 2,745 kj/mol. Some of these electrons are more tightly bound in the atom than others. 3rd ionization energy, 2881 kj ⋅ mol−1. 4th ionization energy, 11600 kj ⋅ mol−1. That is because aluminum has three valence electrons that are located in the outermost shell. Web al,z = 13:1s22s22p63s23p1. Web so without actually providing the ionization energies for all the group 13 elements, they could say that the element has the second highest first ionization energy in its group, which would be aluminum.. Web to draw a successive ionization energy diagram for aluminum, we will use the ionization energy data given on page 60. Web so, this is high, high ionization energy, and that's the general trend across the periodic table. Aluminum is around, group is on group three a. I 1 i_1 i 1 = 578 kj/mol. I i is therefore the. Web the first four ionisation energies of aluminium, for example, are given by. Web in a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make covalent or ionic bonds with each other. An element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a. The first ionization energy is the energy required to remove the outermost (valence). On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13). Web the first four ionisation energies of aluminium, for example, are given by. On the periodic table, first ionization energy generally increases as you move left. Web al,z = 13:1s22s22p63s23p1. The second ionization energy of aluminum is larger than the first, and the third ionization energy is even larger. Now, what about trends up and down the periodic table? This jump corresponds to removal of the core electrons, which are harder to remove than the valence electrons. E(g) → e+(g) +e− energy required=i (2.9.1) (2.9.1) e. I 3 i_3 i 3 = 2,745 kj/mol. Web chemists define the ionization energy ( i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. This jump corresponds to removal of the core electrons, which are harder to remove than the valence electrons. But even. The ionization energy that corresponds to removing an electron from the noble gas configuration would be substantially higher than those before. Aluminum is around, group is on group three a. Web in a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make covalent or ionic bonds with each other. Web ionization. This jump corresponds to removal of the core electrons, which are harder to remove than the valence electrons. As you go from left to right, you go from low ionization energy to high ionization energy. 2nd ionization energy, 1816 kj ⋅ mol−1; Periodic properties of the elements For example, for p, the 5th ie is 6,270, while the 6th ie. Web ionization energy chart of all the elements is given below. I 4 i_4 i 4 = 11,577 kj/mol 4th ionization energy, 11600 kj ⋅ mol−1. I 2 i_2 i 2 = 1,817 kj/mol. Thus, many students find it confusing that, for example, the 5p orbitals fill immediately after the 4d, and immediately before the 6s.the filling order is based. Web ionization energy chart of all the elements is given below. That is because aluminum has three valence electrons that are located in the outermost shell. As you go from left to right, you go from low ionization energy to high ionization energy. Web in a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make covalent or ionic bonds with each other. Web chemists define the ionization energy ( i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. Web x 2+ → x 3+ + e − ionization energy for different elements there is an ionization energy for each successive electron removed. Web the first ionization energy of aluminum is smaller than magnesium. Web each successive ionization energy would be larger in magnitude than the previous one. Web electron configuration for aluminum (al, al3+ ion) aluminum is the 13th element in the periodic table and its symbol is ‘al’. This jump corresponds to removal of the core electrons, which are harder to remove than the valence electrons. 1s 2 2s 2 2p 6 3s 2: In this article, i have discussed in detail how to easily write the complete electron configuration of aluminum. For example, for p, the 5th ie is 6,270, while the 6th ie is 21,200. An element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13). Web al,z = 13:1s22s22p63s23p1.
Question Video Correlation between Ionization Energy and Electron
Electron arrangements
Ionisation Energy AS Level Teaching Resources

Successive Ionisation Energy vigglegiggle

Explaining Successive Ionisation Energies YouTube

12.1 Successive ionisation energies (HL) YouTube

Atomic structure

Atomic structure
Ionization energy Chemistry Steps
Ionization energy Chemistry Steps
Web The First Four Ionisation Energies Of Aluminium, For Example, Are Given By.
On The Periodic Table, First Ionization Energy Generally Increases As You Move Left To Right Across A Period.
I 4 I_4 I 4 = 11,577 Kj/Mol
I I Is Therefore The Energy Required For The Reaction.
Related Post:
