Draw All Resonance Structures For The Carbonate Ion Co32-
Draw All Resonance Structures For The Carbonate Ion Co32- - You do not need to include resonance arrows. Draw the lewis structure of ozone (o3) showing all possible resonance structures if there are any. Do not include overall ion charges or formal charges in your drawing. Remember to indicate formal charges on atoms that have them in each structure. This problem has been solved! Discusses resonance and bond order. Web answer link the answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. Web the lewis structure of carbonate ion can be drawn as follows: Draw one structure per sketcher box, and separate added sketcher boxes with the ↔ symbol. Web the carbonate ion is an example of a molecule for which we can draw three equivalent structures. Web unlike o 3, though, the actual structure of co 32− is an average of three resonance structures. Identify which orbitals overlap to create each bond.openstax™ is a registered trademark,. (b) what is molecular and electron geometry of carbonate ion? Web for the carbonate ion, co 3 2−, co 3 2−, draw all of the resonance structures. Web how to. Determine the formal charge of each atom. The three oxygens are drawn in the shape of a triangle with the carbon at the center of the triangle. Use the concept of resonance to explain structural features of molecules and ions. This problem has been solved! Remember to indicate formal charges on atoms that have them in each structure. You'll get a detailed solution from a subject matter expert that helps you learn core. Each structure has one double bond and two single bonds, suggesting that one of the bonds is shorter than the other two. Web draw the resonance structures of molecules or ions that exhibit delocalization. Remember to indicate formal charges on atoms that have them in. (b) what is molecular and electron geometry of carbonate ion? Identify which orbitals overlap to create each bond. Web draw the resonance structures of molecules or ions that exhibit delocalization. Determine the relative stability of resonance structures using a set of rules. If there are equivalent resonance structures, draw all of them. The correct lewis structure for this ion has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. Remember to indicate formal charges on atoms that have them in each structure. This problem has been solved! You do not need to include resonance arrows. You'll get a detailed solution from a subject matter expert that helps you learn core. Because carbon is the least electronegative element, we place it in the central position: Draw one structure per sketcher box, and separate added sketcher boxes with the ↔ symbol. Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are neutral. Web the lewis structure of carbonate ion can be drawn as follows:. We start with a valid lewis structure and then follow these. Do not include overall ion charges or formal charges in your drawing. Web for the carbonate ion, co 3 2−, co 3 2−, draw all of the resonance structures. Discusses resonance and bond order. Web draw the resonance structures of molecules or ions that exhibit delocalization. The terminal oxygen atoms containing lone pairs act as. Web unlike o 3, though, the actual structure of co 32− is an average of three resonance structures. (a) draw all of them. This video discusses the resonance structure of this polyatomic ion as well as the. You do not need to include resonance arrows. We start with a valid lewis structure and then follow these. For the carbonate ion, co3 2−, draw all of the resonance structures. A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, h 3. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Identify the resonance. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core. This problem has been solved! Use the concept of resonance to explain structural features of molecules and ions. Identify which orbitals overlap to create each bond. Web answer link the answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. This video discusses the resonance structure of this polyatomic ion as well as the. Web draw three resonance structures for carbonate ion, co32, and assign formal charges on all the atoms. Web unlike o 3, though, the actual structure of co 32− is an average of three resonance structures. Web the carbonate ion is an example of a molecule for which we can draw three equivalent structures. Use the concept of resonance to explain structural features of molecules and ions. Web how to draw resonance structure of carbonate ion as with ozone, the carbonate ion’s electronic structure cannot be explained by a single lewis electron structure. The three oxygens are drawn in the shape of a triangle with the carbon at the center of the triangle. Identify which orbitals overlap to create each bond. The correct lewis structure for this ion has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. Web for the carbonate ion, co 3 2−, co 3 2−, draw all of the resonance structures. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, h 3. Draw the lewis structure of ozone (o3) showing all possible resonance structures if there are any. Determine the relative stability of resonance structures using a set of rules.
How To Draw The Lewis Structure of CO3 2 (Carbonate Ion) Chemistry
Solved Draw All Resonance Structures For The Carbonate Io...

CO32 Molecular Geometry, Shape and Bond Angles (Carbonate Ion) YouTube

How to draw the Lewis structure of CO3 2 (Carbonate ion) YouTube

Problem 4.3Explain the structure of CO3^2 (carbonate) ion in termsof

Lewis Structure of the Carbonate Ion (CO32) YouTube

CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2
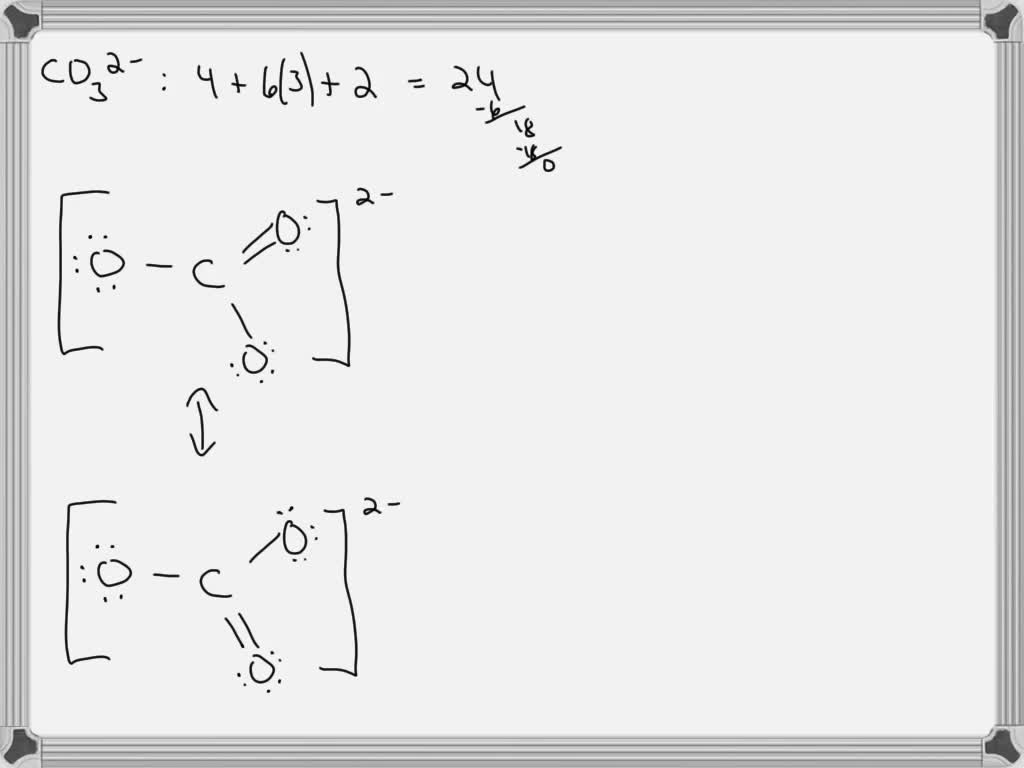
SOLVED For CO32 , carbonate ion, draw the Lewis structure (by

Write the resonance structures of CO3^2 and HCO3^
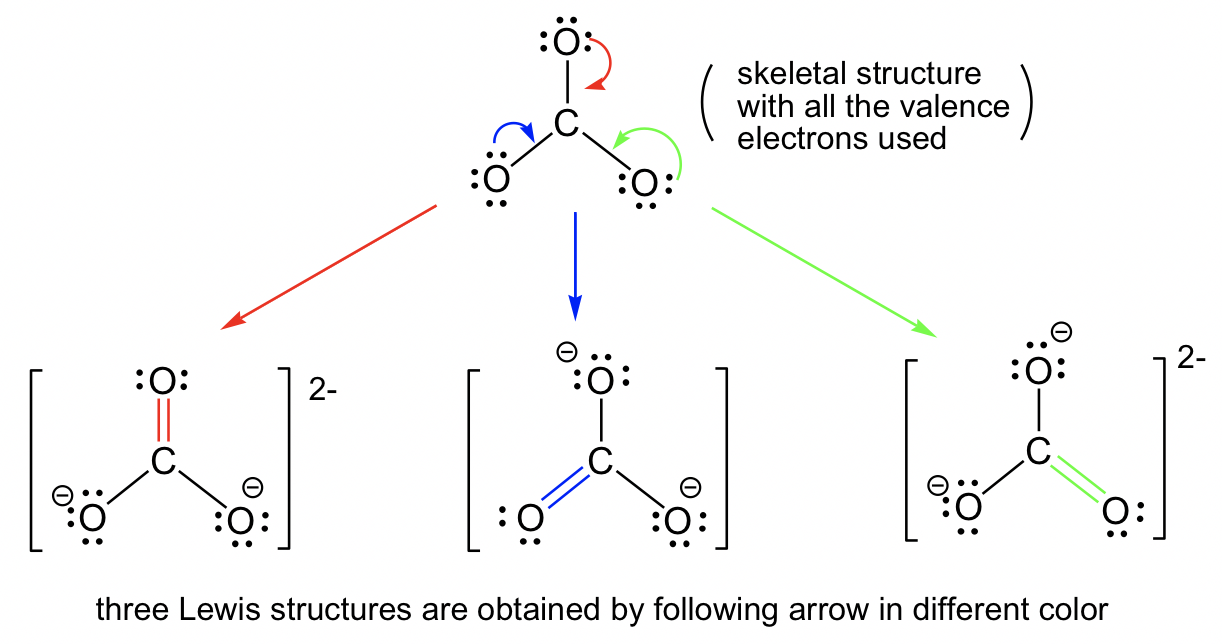
1.3 Resonance Structures Organic Chemistry I
If There Are Equivalent Resonance Structures, Draw All Of Them.
It Has A Total Of 24.
We Start With A Valid Lewis Structure And Then Follow These.
Remember To Indicate Formal Charges On Atoms That Have Them In Each Structure.
Related Post: