Draw All Resonance Structures For The Nitrate Ion No3-
Draw All Resonance Structures For The Nitrate Ion No3- - Therefore, we can draw resonance structures. Web please draw the resonance lewis structures of no3. Magnetic properties of complex ions: Draw another equivalent resonance structure of no₃⁻. Resonance doesn't mean there's a double bond and two. Web draw two resonance structures for the nitrite ion (no 2 −). Draw the resonance structure for the following molecule; Just take note that the only bond moving is the pi (π) bond or in layman's term, the double bond and one of the electron pairs from o atom. Each oxygen atom is bonded to the nitrogen atom, and the molecule has an overall negative charge. Web all the resonance structures are correct since it all follows the octet rule and all have a total number of 24 electrons. The nitrogen atom is quaternized (and thus formally positive) in all the representations. Web draw two resonance structures for the nitrite ion (no 2 −). Total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anion there are one nitrogen atom and three oxygen atoms in the nitrate ion. Draw the resonance structure. Web draw the lewis structures for three resonance forms of the nitrate ion, no3. Just take note that the only bond moving is the pi (π) bond or in layman's term, the double bond and one of the electron pairs from o atom. Include electron lone pairs, and any formal charges. Web in another tutorial, we learn how to draw. Therefore, we can draw resonance structures. Web science chemistry chemistry questions and answers 4. This question hasn't been solved yet! Include electron lone pairs, and any formal charges. Magnetic properties of complex ions 8m. Select draw rings more erase n o this problem has been solved! Draw two resonance structures for the nitrosonium. Web please draw the resonance lewis structures of no3. Web chemistry questions and answers. Total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anion there are one nitrogen atom and three oxygen atoms. Resonance structures of no3 (. Web science chemistry chemistry questions and answers 4. Web please draw the resonance lewis structures of no3. Magnetic properties of complex ions: You can decide this from looking about whether atoms have lone pairs or double bonds. Organic chemistry resonance what is resonance? Nitrate anion possesses 3 formal charges on its atoms. Draw two resonance structures for the nitrosonium. Crystal field theory summary 5m. • include all valence lone pairs in your answer. • do not include overall ion charges or formal charges in your drawing. Draw all resonance structures for the nitrate ion, no3 • explicitly draw all h atoms. The nitrogen atom is quaternized (and thus formally positive) in all the representations. Web in another tutorial, we learn how to draw resonance structures of nitrate ion. You'll get a detailed solution. This problem has been solved! Web each of stable resonance structure of nitrate ion is a lewis structure. You can decide this from looking about whether atoms have lone pairs or double bonds. Web all the resonance structures are correct since it all follows the octet rule and all have a total number of 24 electrons. You can do this. Send to expert send to expert send to expert done loading. You can do this by (1) drawing the electron configuration per element or (2) consulting your periodic table. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw all resonance structures for the nitrate ion, no3 • explicitly draw all h atoms.. Resonance doesn't mean there's a double bond and two. Web each of stable resonance structure of nitrate ion is a lewis structure. Web draw the lewis structures for three resonance forms of the nitrate ion, no3. Draw two resonance structures for the nitrosonium. Nitrate anion possesses 3 formal charges on its atoms. Web science chemistry chemistry questions and answers a lewis structure for the nitrate ion (no₃⁻) is shown on the left below. Web all the resonance structures are correct since it all follows the octet rule and all have a total number of 24 electrons. • do not include overall ion charges or formal charges in your drawing. Crystal field theory summary 5m. Web draw two resonance structures for the nitrite ion (no 2 −). Total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anion there are one nitrogen atom and three oxygen atoms in the nitrate ion. Select draw rings more erase n o this problem has been solved! Resonance structures of no3 (. Therefore, we can draw resonance structures. (ii) draw the resonating structure of. Magnetic properties of complex ions 8m. Resonance doesn't mean there's a double bond and two. Each oxygen atom is bonded to the nitrogen atom, and the molecule has an overall negative charge. Send to expert send to expert send to expert done loading. Dec 22, 2015 by exchanging the electron pairs around the oxygens. If there are equivalent resonance structures, draw all of them.
Lewis dot structure of NO3 ion Nitrate ion lewis structure YouTube
[Solved] Draw all valid Lewis structures for the nitrate ion, showing

Lewis dot structure of the nitrate ion NO3 YouTube
[Solved] 2. Draw four resonance forms of the nitrate ion NO3
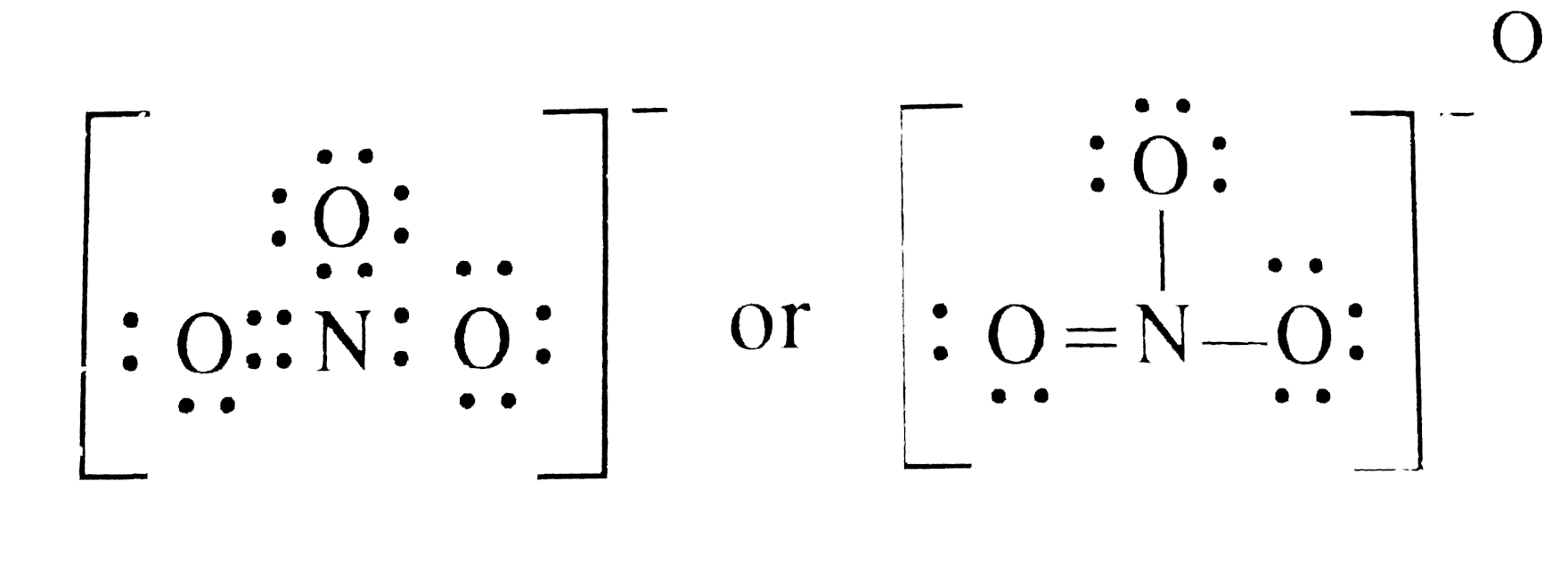
How To Draw The Lewis Dot Structure For No3 Nitrate Ion
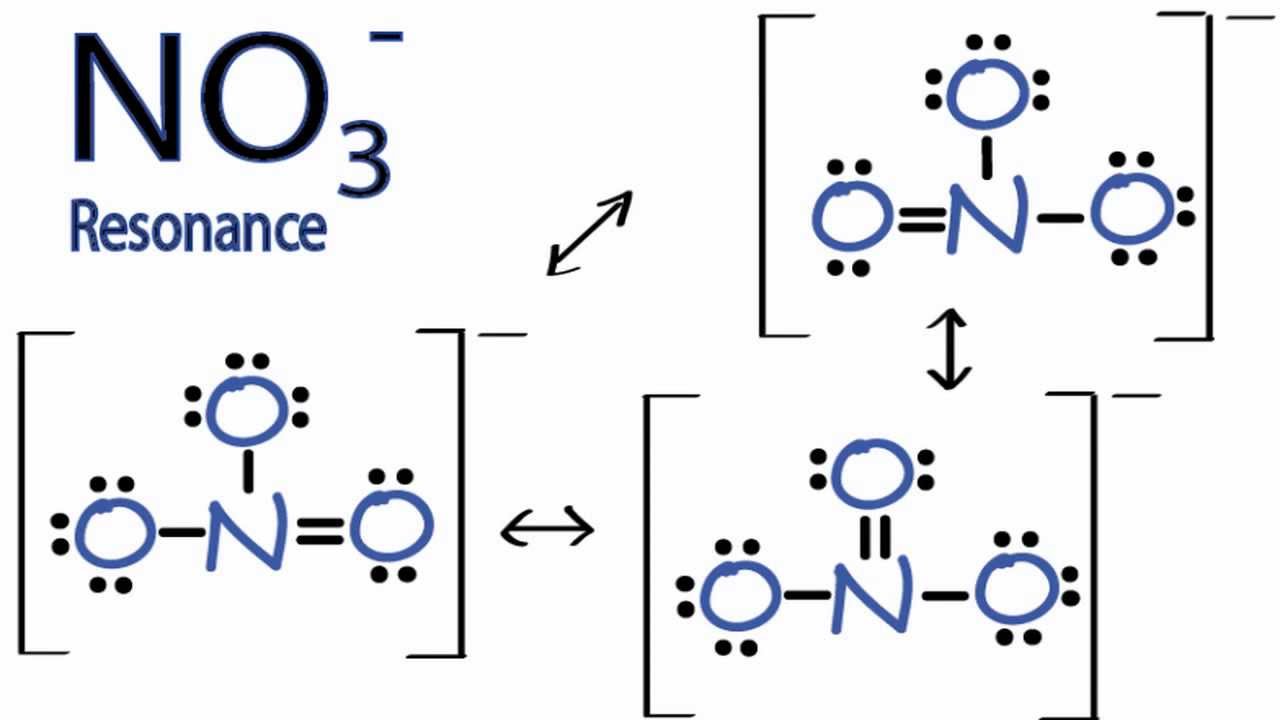
Resonance Structures for NO3 (Nitrate Ion) YouTube
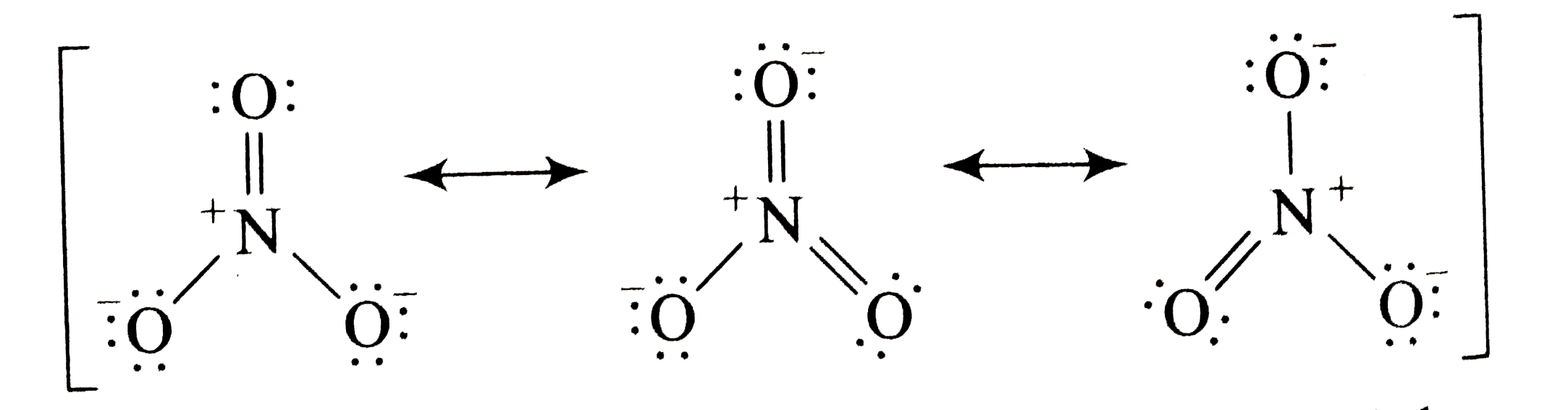
How many resonance structures can be drawn for the nitrate ion, NO3^(
[Solved] Draw Lewis structure(s) for the nitrate ion ( NO 3 ). If
[Solved] 2. Draw four resonance forms of the nitrate ion NO3
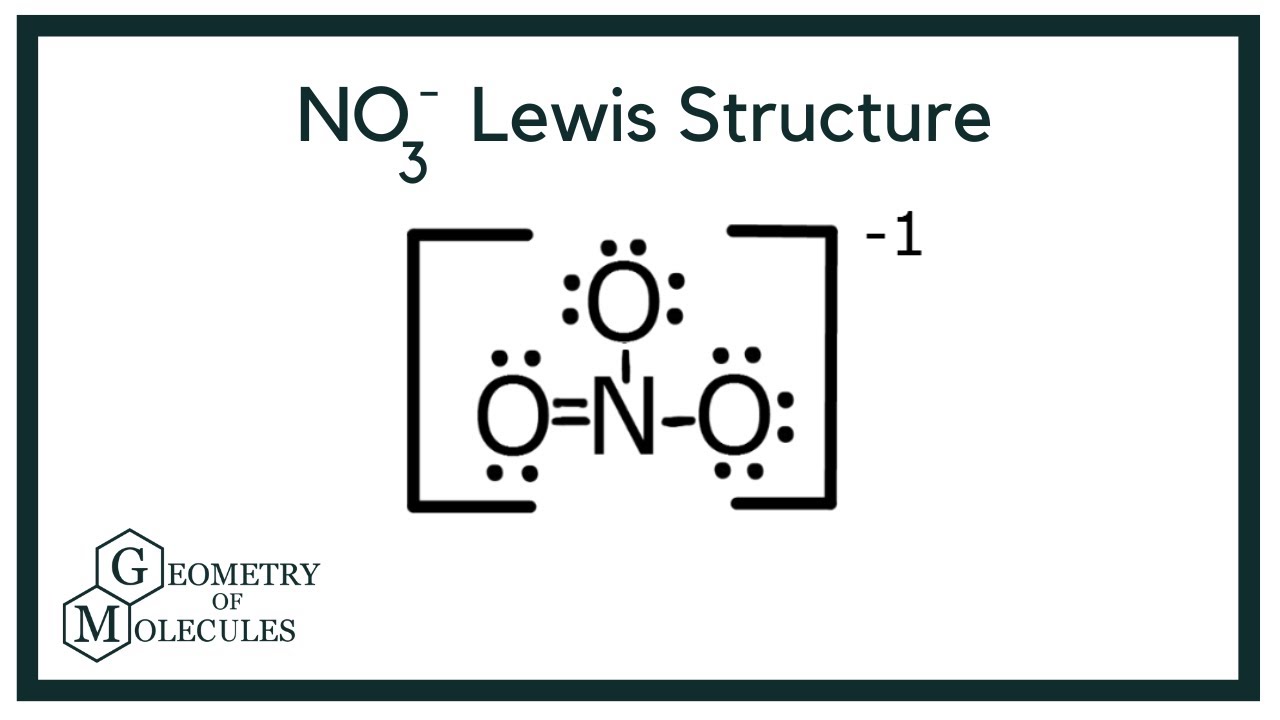
NO3 Lewis Structure Draw Lewis Dot Structure of Nitrate Ion YouTube
First Is You Need To Know The Number Of Valence Electrons.
• Include All Valence Lone Pairs In Your Answer.
Web 1 Answer Anor277 · Ernest Z.
Include Electron Lone Pairs, And Any Formal Charges.
Related Post: