Draw All Resonance Structures For The Nitryl Chloride Molecule No2Cl
Draw All Resonance Structures For The Nitryl Chloride Molecule No2Cl - In each shared pair the carbon atom owns ____ electron. Ch2cl2(g) + hcl(g) draw the lewis structures for all reactant and… Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Draw lewis structures for the nine isomers having molecular formula c3h,0, with all atoms having a… If there are equivalent resonance structures, draw all of them. Web remember the following rules for drawing resonance structures: Draw lewis structure(s) for the nitryl chloride molecule (no2cl). Matter and change 1st edition isbn: Draw lewis structure(s) for the nitronium ion (no2+). Draw lewis structure (s) showing all possible equivalent resonance forms for the nitryl chloride molecule ( noci). Draw all resonance structures for the nitryl chloride molecule, no2cl. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Ch2cl2(g) + hcl(g) draw the lewis structures for all reactant and… Explicitly draw all h atoms. Web expert answer transcribed image text: Draw one structure per sketcher box, and separate added sketcher boxes with the ↔ symbol. Web get the detailed answer: Web draw resonance structure for the following molecule. Draw all resonance structures for the nitryl chloride molecule, no2cl. (b) include all valence lone pairs in your answer. • explicitly draw all h atoms. Draw a lewis structure (including all lone pair electrons) and calculate the formal charge (fc) of each atom of nitrosyl chloride (clno) fc on cl fc on n fc on o. Web for nitryl chloride (no2cl), the total number of valence electrons available is 24 (5 from nitrogen + 7 from chlorine + 6. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Do not show any n charges in your drawings. Do not show ion charges in your drawings.per sketcher box, and separate added sketcher boxes with the ↔ symbol. (d) do not draw double bonds to oxygen unless they are needed. Draw lewis structure(s) for the nitryl chloride molecule (no2cl). There are three resonance structures for no2cl. (c) do not include overall ion charges or formal charges in your drawing. Draw lewis structure (s) showing all possible equivalent resonance forms for the nitryl chloride molecule ( noci). There is along with one flooring. Electrons can only be moved from π \pi π bonds or lone pairs of p p p orbitals. Resonance structures are sets of lewis structures that describe the delocalization of electrons in a… q: The central atom and double bond are present, along with four number of electrons. (a) explicitly draw all h atoms. (b) include all valence lone pairs. Matter and change 1st edition isbn: Draw all resonance structures for the nitryl chloride molecule, no2cl. Resonance structures are sets of lewis structures that describe the delocalization of electrons in a… q: Draw one structure per sketcher box, and separate added sketcher boxes with the ↔ symbol. Web science chemistry draw all resonance structures for the nitryl chloride molecule, no2cl. (c) do not include overall ion charges or formal charges in your drawing. Draw one structure per sketcher box, and separate added sketcher boxes with the ↔ symbol. Draw a lewis structure (including all lone pair electrons) and calculate the formal charge (fc) of each atom of nitrosyl chloride (clno) fc on cl fc on n fc on o. Web. Matter and change 1st edition isbn: Do not include overall ion charges or formal charges in your drawing. To understand its chemical and physical properties, one need. Show all atoms, bonds, lone pairs, and formal charges. Explicitly draw all h atoms. (b) include all valence lone pairs in your answer. Web get the detailed answer: Draw one structure per sketcher box, and separate any added sketcher boxes with the ↔ symbol. Atoms should not be moved. • do not include overall ion charges or formal charges in your drawing. The central atom and double bond are present, along with four number of electrons. Show all atoms, bonds, lone pairs, and formal charges. Do not include overall ion charges or formal charges in your drawing. Web science chemistry chemistry questions and answers draw lewis structure (s) showing all possible equivalent resonance forms for the nitryl chloride molecule (no2cl draw one structure per sketcher box, and separate any added sketcher boxes with the symbol. Web chemistry chemistry questions and answers draw all equivalent resonance structures for the nitryl chloride molecule, no2cl. These 24 electrons are required to form a single no2cl molecule. We are going to talk about the lewis structure of n. (a) explicitly draw all h atoms. Draw lewis structure(s) for the nitryl chloride molecule (no2cl). Draw all resonance structures for the nitryl chloride molecule, no2cl. Draw all resonance structures for the nitryl chloride molecule, no2cl. Do not show ion charges in your drawings.per sketcher box, and separate added sketcher boxes with the ↔ symbol. To understand its chemical and physical properties, one need. There are three resonance structures for no2cl. Web draw resonance structure for the following molecule. There is along with one flooring.
In nitryl chloride, NO2Cl, there is no oxygenoxygen bond Write a Lewis
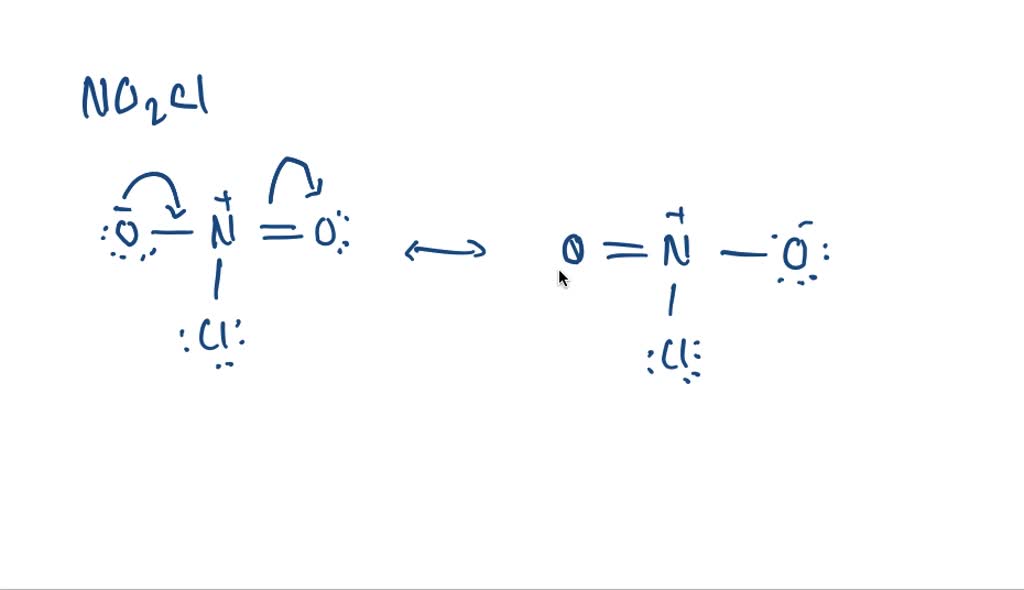
SOLVEDThe formula for nitryl chloride is NO2 Cl. Draw the Lewis

NO2Cl Lewis Structure How to Draw the Lewis Structure for NO2Cl YouTube
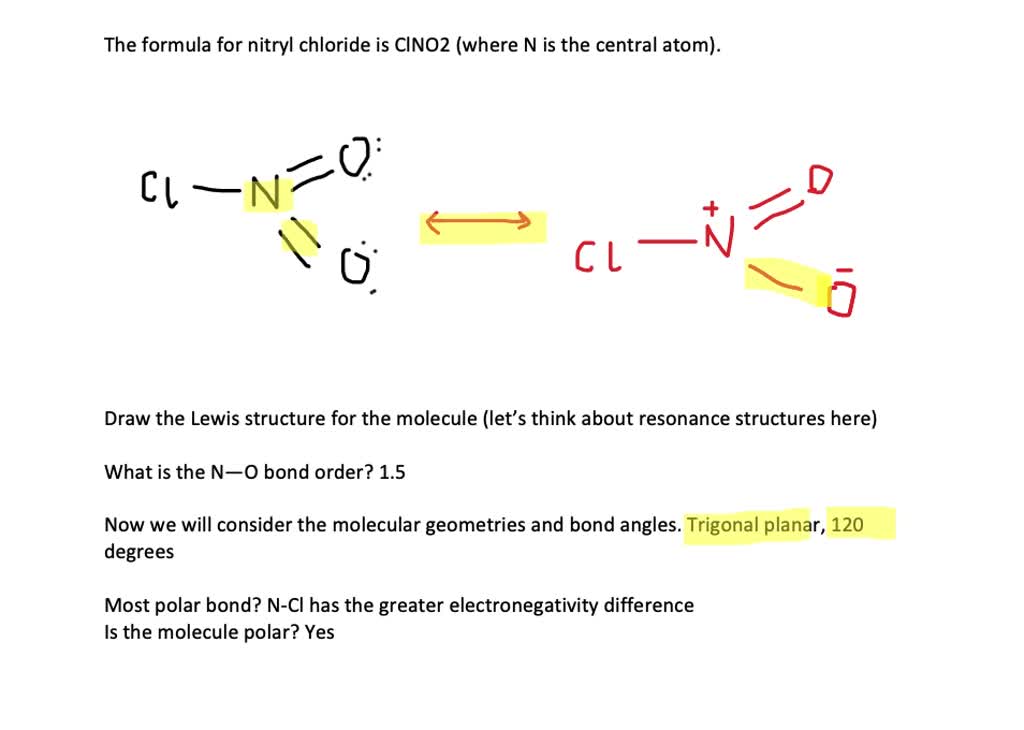
SOLVEDThe formula for nitryl chloride is CINO 2. (a) Draw the Lewis
Solved Draw All Resonance Structures For The Nitryl Chlor...
Solved Draw ail resonance structures for the nitryl chloride
Solved Draw all resonance structures for the nitryl chloride

NO2Cl Lewis Structure, Molecular Geometry, Hybridization, and Polarity

NO2Cl Lewis Structure (Nitryl Chloride) YouTube

NO2Cl Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Atoms Should Not Be Moved.
Draw One Structure Per Sketcher.
The Lone Pair On N Is Delocalized In All Three Structures.
The First One Has A Double Bond Between N And The O Atom, The Second One Has A Double Bond Between N And The Other O Atom, And The Third One Has A Double Bond Between N And The Cl Atom.
Related Post: