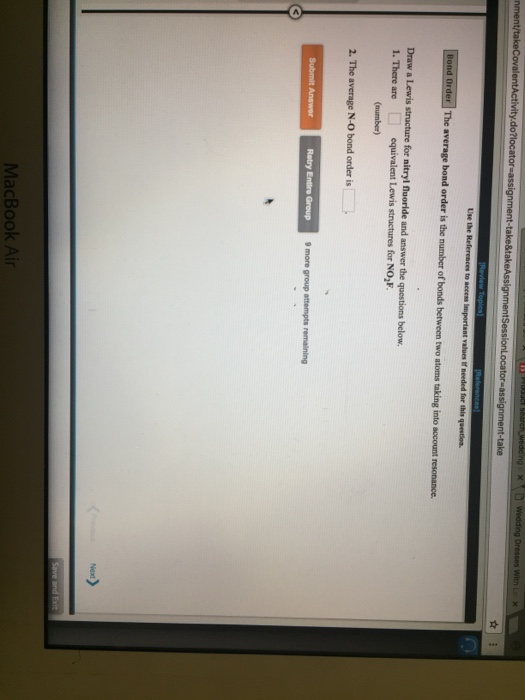
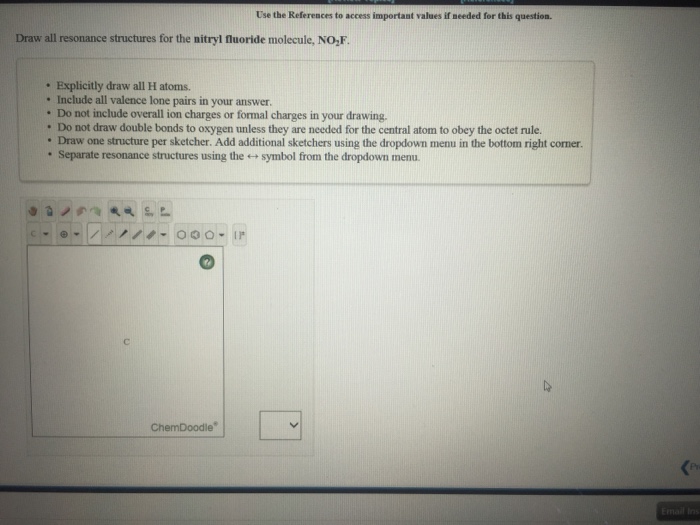
Draw All Resonance Structures For The Nitryl Fluoride Molecule No2F
Draw All Resonance Structures For The Nitryl Fluoride Molecule No2F - Do not include overall ion charges or formal charges in your drawing. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Do not show any ion charges in your drawings. Draw the lewis dot structure of nitryl fluoride: Provide the oxidation state and the formal charge of the central n atom. So to start off our drawings, we're going to have to know how many electrons are going to go into our image. Demonstrate that this molecule is stabilized by resonance. The lewis structure helps us. We have 5 valence electrons for nitrogen, 6 for each oxygen atom (total 12), and 7 for fluorine, adding up to 24 electrons. Draw all resonance structures for the nitryl fluoride molecule, no_2f. (a) explicitly draw all h atoms. Provide the oxidation state and the formal charge of the central n atom. Draw one structure per sketcher box, and separate any added sketcher boxes with the ↔ symbol. Web draw lewis structure(s) showing all possible equivalent resonance forms for the nitryl fluoride molecule ( no2f ). Web draw all resonance structures for the. Draw all resonance structures for the nitryl fluoride molecule no2f. • do not include overall ion charges or formal charges in your drawing. Do not include overall ion charges or formal charges in your drawing. • include all valence lone pairs in your answer. (a) explicitly draw all h atoms. Draw the lewis dot structure of nitryl fluoride: Do not include overall ion charges or formal charges in your drawing. Provide the oxidation state and the formal charge of the central n atom. The oxygen atom with the negative charge can. There are equivalent lewis structures for no_2f. Do not show any ion charges in your drawings. Web there will be two resonance structures of nitryl fluoride as any oxygen atom can share its electron pair. The lewis structure helps us. Do not include overall ion charges or formal charges in your drawing. Draw one structure per sketcher box, and separate any added sketcher boxes with the ↔. Web these structures are called resonance structures. (b) include all valence lone pairs in your answer. (d) do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Web draw all resonance structures for the nitryl fluoride molecule, no2f. Draw one structure per sketcher box, and separate any added sketcher boxes. The oxygen atom with the negative charge can. (c) do not include overall ion charges or formal charges in your drawing. Draw one structure per sketcher box, and separate any added sketcher boxes with the ↔ symbol. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Create your account view this answer the. 1 become a study.com member to unlock this answer! In the case of no2f, nitrogen (n) is the central atom bonded to two oxygen (o) atoms, one fluorine (f) atom, and one lone pair of electrons. Nitrogen (n) is the least electronegative atom and goes at the center of this structure. The resonance structures for no2f involve a double bond. Demonstrate that this molecule is stabilized by resonance. Web draw all resonance structures for the nitryl fluoride molecule, no2f. This problem has been solved! Web science chemistry chemistry questions and answers draw lewis structure (s) showing all possible equivalent resonance forms for the nitryl chloride molecule (no2cl draw one structure per sketcher box, and separate any added sketcher boxes with. The resonance structures for no2f involve a double bond between nitrogen and oxygen or a single bond between nitrogen and oxygen with a lone pair on nitrogen. Explicitly draw all h atoms. Draw one structure per sketcher box, and separate any added sketcher boxes with the ↔ symbol. Web draw all resonance structures for the nitryl fluoride molecule, no₂f. Explicitly. Web draw all resonance structures for the nitryl fluoride molecule, n o 2 f. Resonance can be defined as a method in which the delocalization of electrons is represented. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (d) do not draw double bonds to oxygen unless they. There are equivalent lewis structures for no_2f. It shows the arrangement of atoms and the sharing of electrons between them. Explicitly draw all h atoms. Draw the lewis dot structure of nitryl fluoride: • include all valence lone pairs in your answer. • do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web there will be two resonance structures of nitryl fluoride as any oxygen atom can share its electron pair. The oxygen atom with the negative charge can. Explicitly draw all h atoms. Web draw all resonance structures for the nitryl fluoride molecule, no2f. Web these structures are called resonance structures. So to start off our drawings, we're going to have to know how many electrons are going to go into our image. Draw one structure per sketcher box, and separate any added sketcher boxes with the ↔ symbol. Web draw all resonance structures for the nitryl fluoride molecule, no2f. (c) do not include overall ion charges or formal charges in your drawing.Solved Draw a Lewis structure for nitryl fluoride and answer
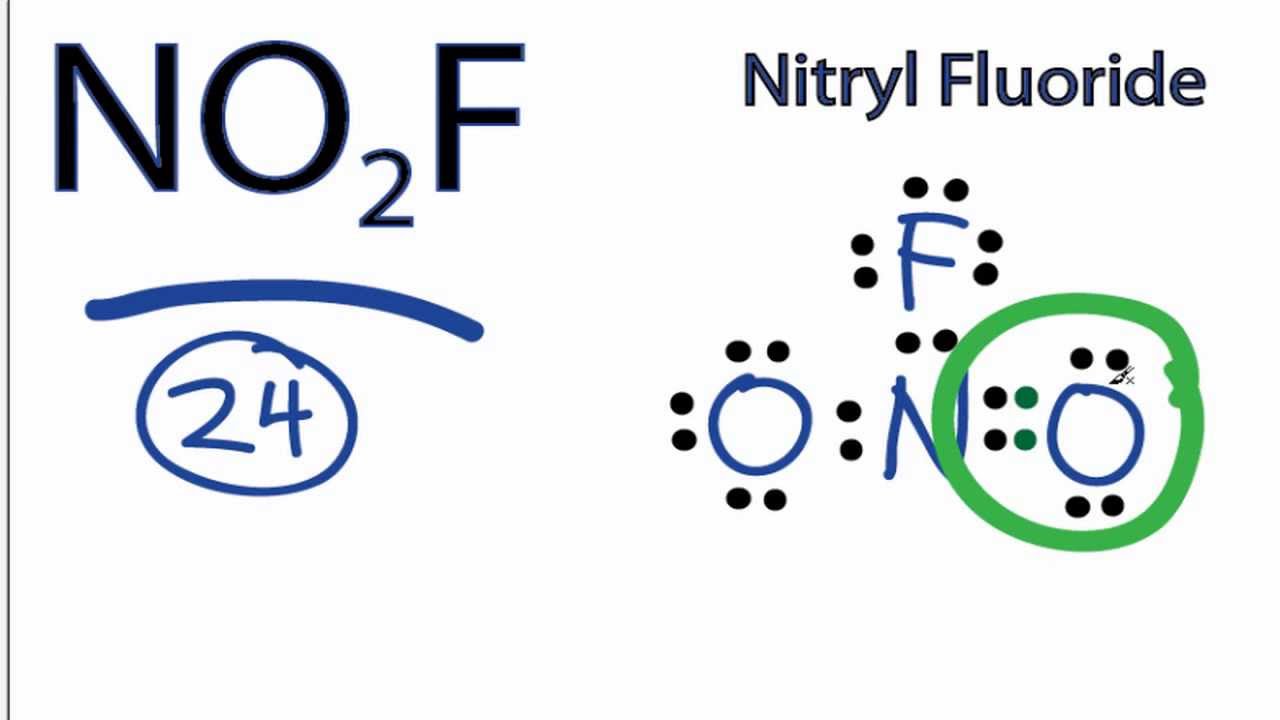
NO2F Lewis Structure How to Draw the Lewis Structure for NO2F YouTube

See Draw All Resonance Structures For The Nitryl Fluoride Molecule No2f

Draw all resonance structures for the nitryl fluoride molecule, NO2F.(a

NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity
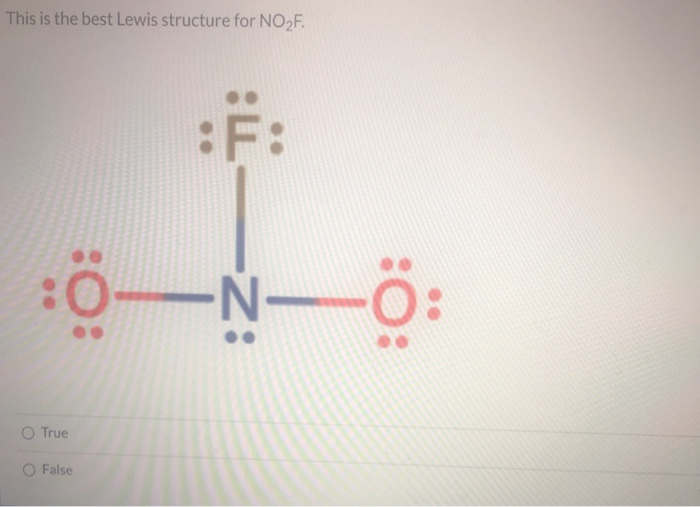
Lewis Structure No2f

NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity

What are the resonance structure of nitryl fluoride? Quizlet
Solved Draw all resonance structures for the nitryl fluoride
Solved Draw all resonance structures for the nitryl chloride
Explicitly Draw All H Atoms.
Draw Lewis Structure (S) Showing All Possible Equivalent Resonance Forms For The Nitryl Fluoride Molecule ( No2F ).
• Do Not Include Overall Ion Charges Or Formal Charges In Your Drawing • Do Not Draw Double Bonds To Oxygen Unless They Are Needed For The Central This Problem Has Been Solved!
Web Draw All Resonance Structures For The Nitryl Fluoride Molecule, No2F.
Related Post: