Draw Five Protons In The Nucleus Of The Atom
Draw Five Protons In The Nucleus Of The Atom - Label them with their charge. What element is represented by the diagram Protons are found inside the nucleus at the center of the atom, and they give the nucleus a positive charge. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. Label them with their charge. To draw five protons in the nucleus of an atom, you can represent them as individual positively charged particles. Electrons are usually represented by a dot or cross. Protons and neutrons are in the center of the atom, making up the nucleus. Draw two electrons in the first energy level and label them with their charge. Its value determines the identity of the atom. Draw three electrons in the second energy level and label them with their charge. (there are also neutrons in the nucleus, but they have no electric charge.) Counting protons, electrons, and neutrons science > chemistry library > If scientists count four protons in an atom, they know it's a beryllium atom. Draw two electrons in the first energy level and. Web atoms are made of extremely tiny particles called protons, neutrons, and electrons. This is the defining trait of an element: Draw 3 electrons in the 2 nd energy level and label with their charge. Counting protons, electrons, and neutrons science > chemistry library > Draw 2 electrons in the 1st stenergy level and label with their charge. Draw six neutrons in the nucleus of the atom 3. Add up to two electrons to the first electron shell. Atomic structure 1 draw five protons in the nucleus of the atom. Students are asked to sketch each part of the atom w/charge. Its value determines the identity of the atom. Name atomic basics part a: Web introduction to chemistry preparing to study chemistry elements and atoms average atomic mass worked example: Draw six neutrons in the nucleus of the atom. Label them with their charge. Web atoms are made of extremely tiny particles called protons, neutrons, and electrons. Draw two electrons in the first energy level and label them with their charge. Electrons have a negative charge. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Draw five protons in the nucleus of the atom. Draw six neutrons in the nucleus of the atom. Draw two electrons in the first energy level and label them with their charge. Label them with their charge. The nucleus (plural, nuclei) is a positively charged region at the center of the atom. Draw six neutrons in the nucleus of the atom. Draw six neutrons in the nucleus of the atom. Name atomic basics part a: Draw three electrons in the second energy level and label them with their charge. 1 for hydrogen, 6 for carbon, 15 for phosphorus, and so on; Draw two electrons in the first energy level and label them with their charge. Identifying isotopes and ions isotope composition: Inside the circle, draw five small dots to represent the protons. Draw five protons in the nucleus of the atom. Web atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Draw five protons in the nucleus of the atom. Draw six neutrons in the nucleus of the atom. Atomic weight calculation the mole and avogadro's number atomic number, mass number, and isotopes worked example: Electrons are usually represented by a dot or cross. Web atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Draw five protons in the nucleus of the atom. Protons and neutrons are in the center of the atom, making. Web introduction to chemistry preparing to study chemistry elements and atoms average atomic mass worked example: Draw five protons in the nucleus of the atom. Draw five protons in the nucleus of the atom. Draw another circle around the first shell. Draw a circle around the nucleus. Draw six neutrons in the nucleus of the atom. Web the protons and neutrons are found in the nucleus close nucleus the central part of an atom. Draw two electrons in the first energy level and label them with their charge. Label them with their charge. The nucleus (plural, nuclei) is a positively charged region at the center of the atom. Draw two electrons in the first energy level and label them with their charge. Label them with their charge. Protons and neutrons are in the center of the atom, making up the nucleus. Draw two electrons in the first energy level and label them with their charge. Add up to two electrons to the first electron shell. Its value determines the identity of the atom. And the weight of these protons, each proton is one atomic mass unit, and we'll talk more about how that relates to kilograms. Its value determines the identity of the atom. Label them with their charge. Web the number of protons in the nucleus of an atom is its atomic number (z). The particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric charge.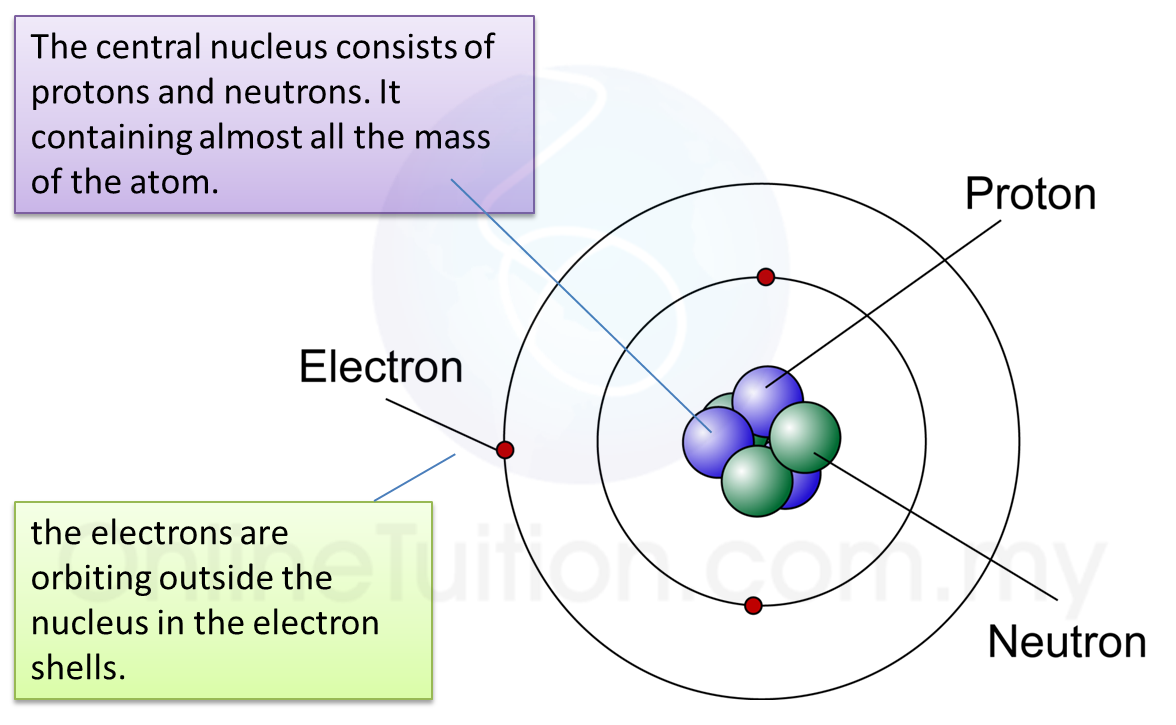
(A) The composition of the Nucleus SPM Science

draw five protons in the nucleus of the atom conversionvanrentalatlanta
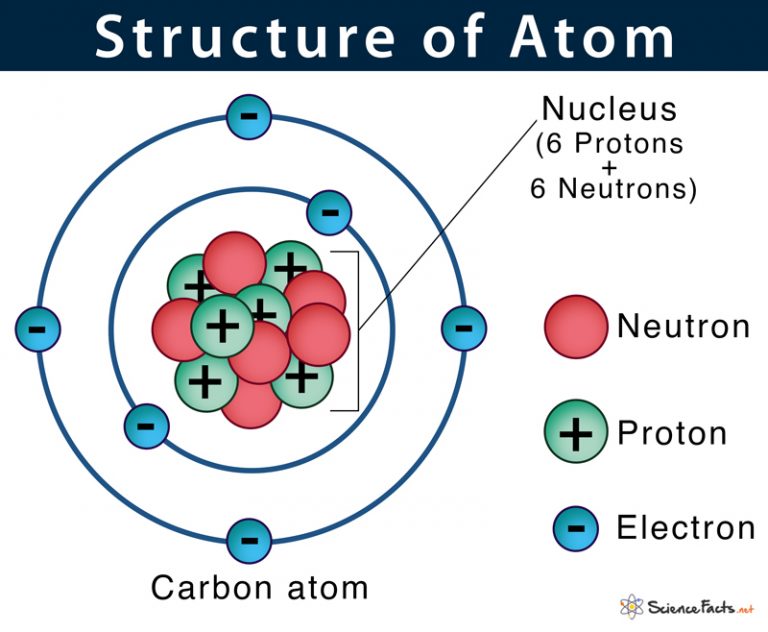
Atom Definition, Structure & Parts with Labeled Diagram

Atomic Nucleus Definition, Structure & Parts with Diagram

Atomic Structure Physical Science For Dummies
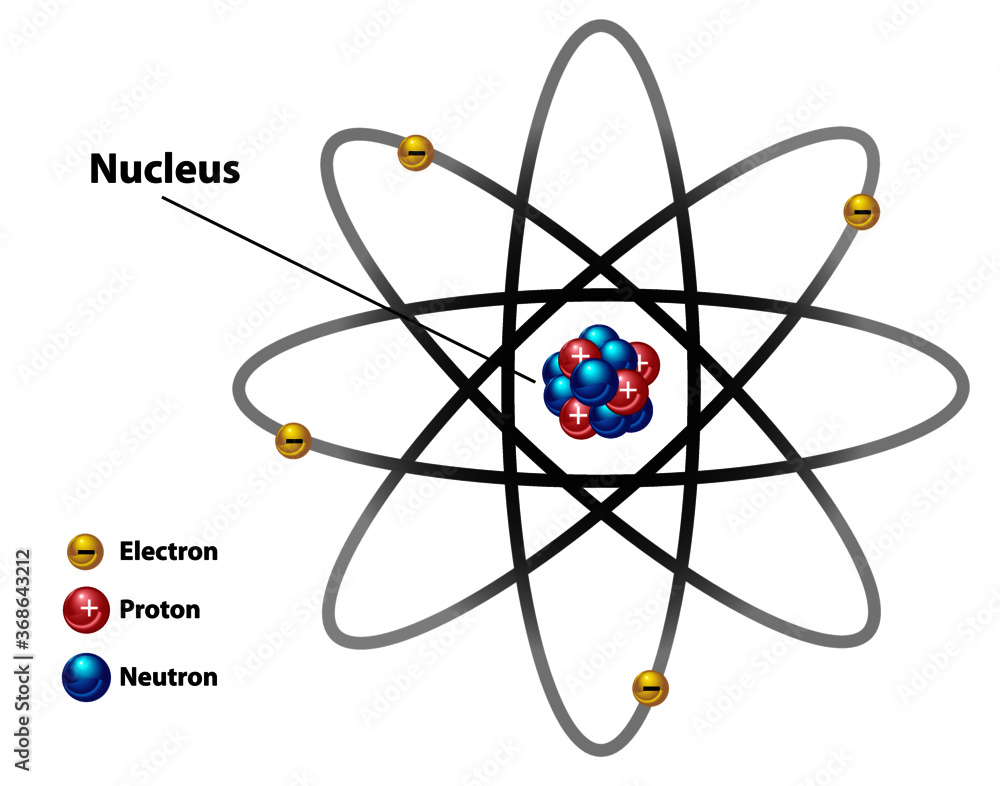
Atomic nucleus diagram labeled with electron, proton, and neutron

Protons In An Atom

draw five protons in the nucleus of the atom errolboatner
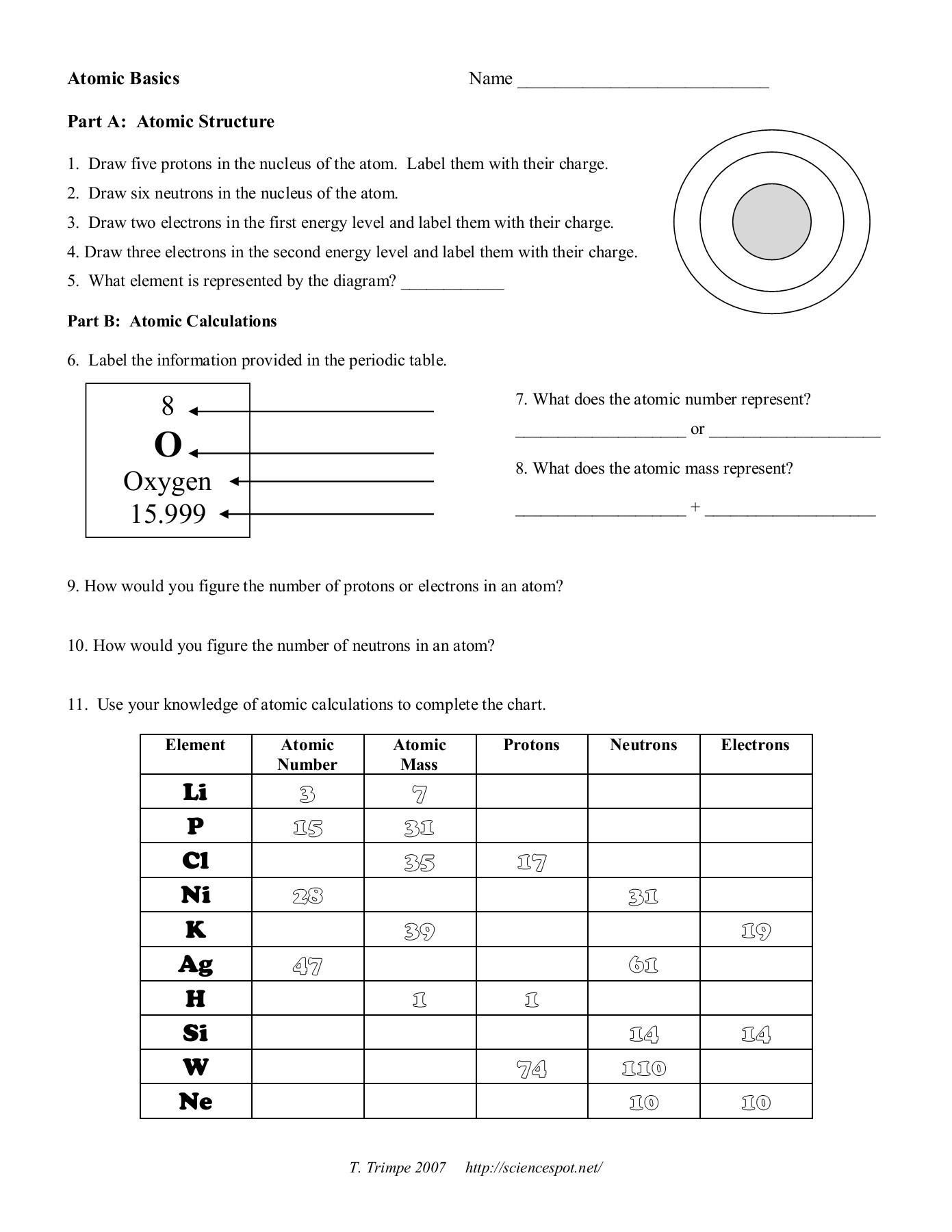
draw five protons in the nucleus of the atom

draw five protons in the nucleus of the atom
It Contains Protons And Neutrons, And Has Most Of The Mass Of The Atom.
Label Them With Their Charge.
Protons Have A Positive Charge.
Web Introduction To Chemistry Preparing To Study Chemistry Elements And Atoms Average Atomic Mass Worked Example:
Related Post: