Draw Nitrogen Atom
Draw Nitrogen Atom - After this, we need to draw the sketch of the molecule with only single bonds. The first two electrons go into the first energy level. Web draw a lewis electron dot diagram for an atom or a monatomic ion. This is how you draw a bohr diagram. [a] nitrogen’s protons and neutrons go into the nucleus. Questions tips & thanks want to join the conversation? Web the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Let’s draw and understand this lewis dot structure step by step. This problem has been solved! As we can see, all the atoms inside the nf3 molecule have the least possible formal charge values. Draw a small circle, inside that circle, write p = 7 and n = 7 the first outer shell holds 2 electrons so draw, 2 electrons on the circle then draw the second shell, which can contain 8 electrons however, you only require 5. Web look for the total number of bonds forming: You'll get a detailed solution from a. Let’s draw and understand this lewis dot structure step by step. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The following image attached can explain this more clearly, we can see after drawing the sketch, remaining electrons are given around the atoms. Web this is known as the octet rule which states that, with the exception of. Nitrogen has 2 electrons in its first shell and 5 in its second. Web draw a lewis electron dot diagram for an atom or a monatomic ion. As we can see, all the atoms inside the nf3 molecule have the least possible formal charge values. Each hydrogen atom has one bond. To obtain an octet, these atoms form three covalent. Top voted christine marie 8 years ago why can't we assume that nitrogen has h bonds (as we did with carbon in the previous video) instead of lone pairs? Web steps to draw the bohr model of nitrogen atom 1. I show you where nitrogen is on the periodic table and how to determine how many valence electrons it has.. Examples of some neutral atoms and their electron configurations are shown in figure 3 3. Therefore, we have attained our most perfect lewis structure diagram. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Each oxygen atom has two bonds. The electronic configuration of nitrogen is. The electronic configuration of nitrogen is 1 s2 2 s2 2 p3. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The following image attached can explain this more clearly, we can see after drawing the sketch, remaining electrons are given around the atoms. Examples of some neutral atoms and their electron configurations are shown in figure 3. The first two electrons go into the first energy level. Web this is known as the octet rule which states that, with the exception of the innermost shell, atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. In this video we'll look at the atomic structure and bohr model for the. Web look for the total number of bonds forming: Find the number of protons, electrons, and neutrons in the nitrogen atom protons are the positively charged particles. Therefore, we have attained our most perfect lewis structure diagram. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Each hydrogen atom has one bond. Web lewis structure of no (or nitric oxide) contains one double bond between the nitrogen (n) atom and oxygen (o) atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Web look for the total number of bonds forming: Questions tips & thanks want to join the conversation? Nitrogen will. Each hydrogen atom has one bond. Questions tips & thanks want to join the conversation? I show you where nitrogen is on the periodic table and how to determine how many valence electrons it has. There are many things to. • ( 8 votes) upvote flag prateek patil 8 years ago no, we can't. Examples of some neutral atoms and their electron configurations are shown in figure 3 3. I show you where nitrogen is on the periodic table and how to determine how many valence electrons it has. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web look for the total number of bonds forming: Three single covalent bonds between each oxygen and hydrogen atom. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Questions tips & thanks want to join the conversation? [a] nitrogen’s protons and neutrons go into the nucleus. These three electrons have unpaired spins. So one of the nitrogen is the middle atom. • ( 8 votes) upvote flag prateek patil 8 years ago no, we can't. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. To obtain an octet, these atoms form three covalent bonds, as in nh (ammonia). Web steps to draw the bohr model of nitrogen atom 1.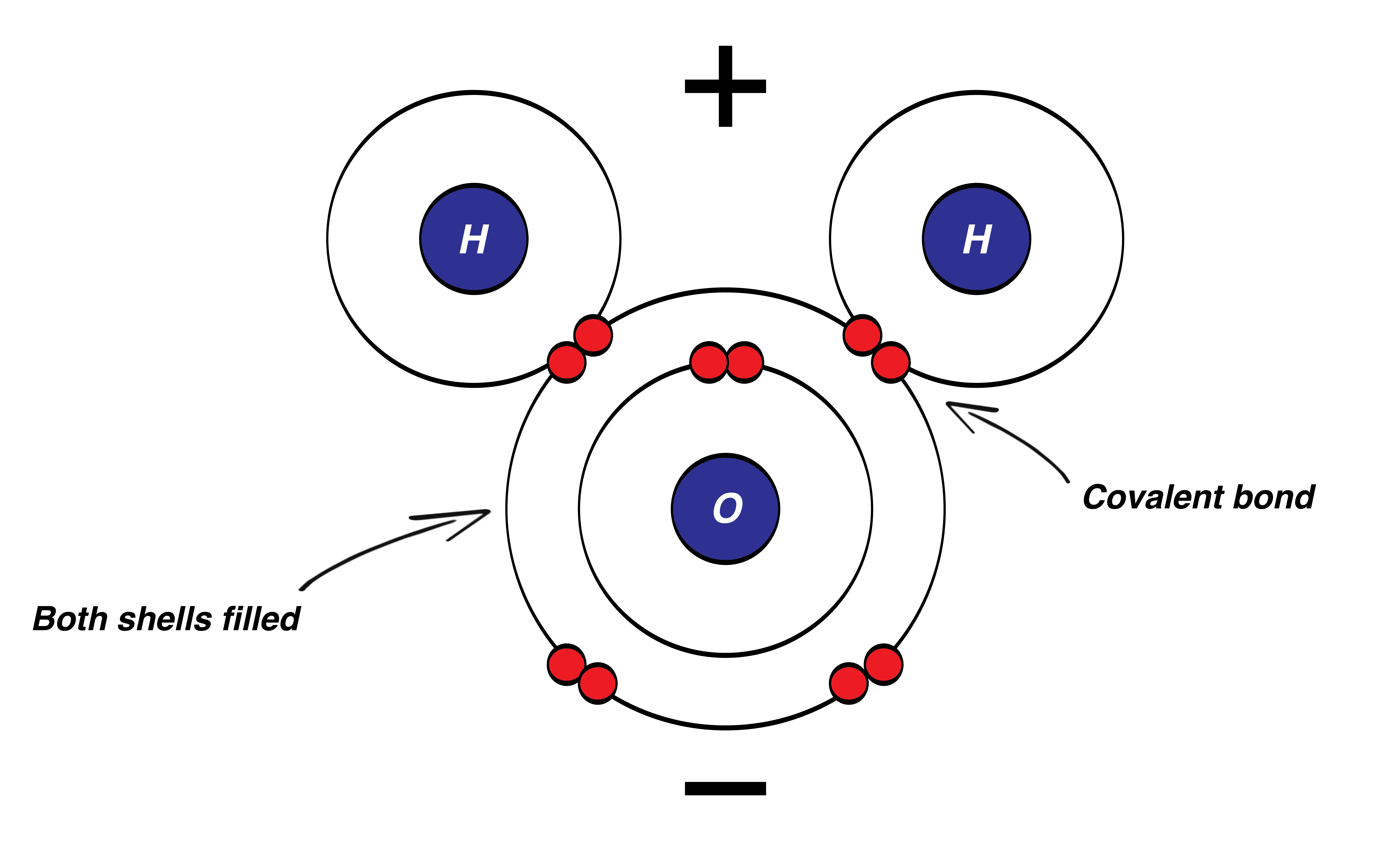
Bohr Rutherford Diagram For Nitrogen
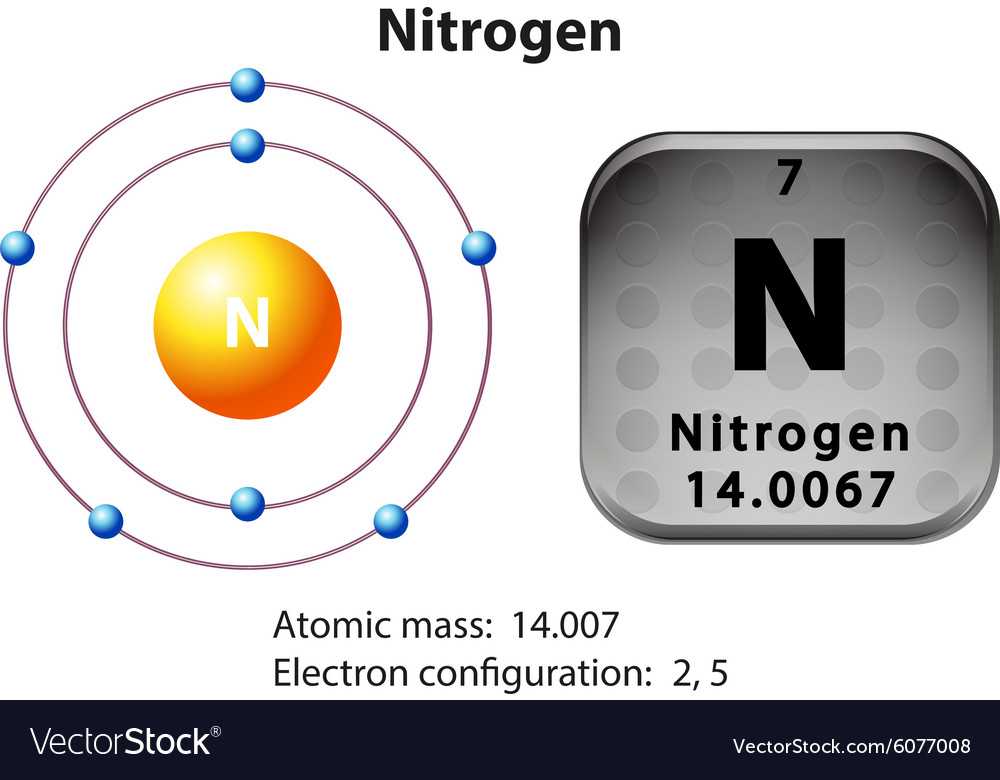
Symbol and electron diagram for nitrogen Vector Image

Diagram representation of the element nitrogen Vector Image

Nitrogen atom heavykesil
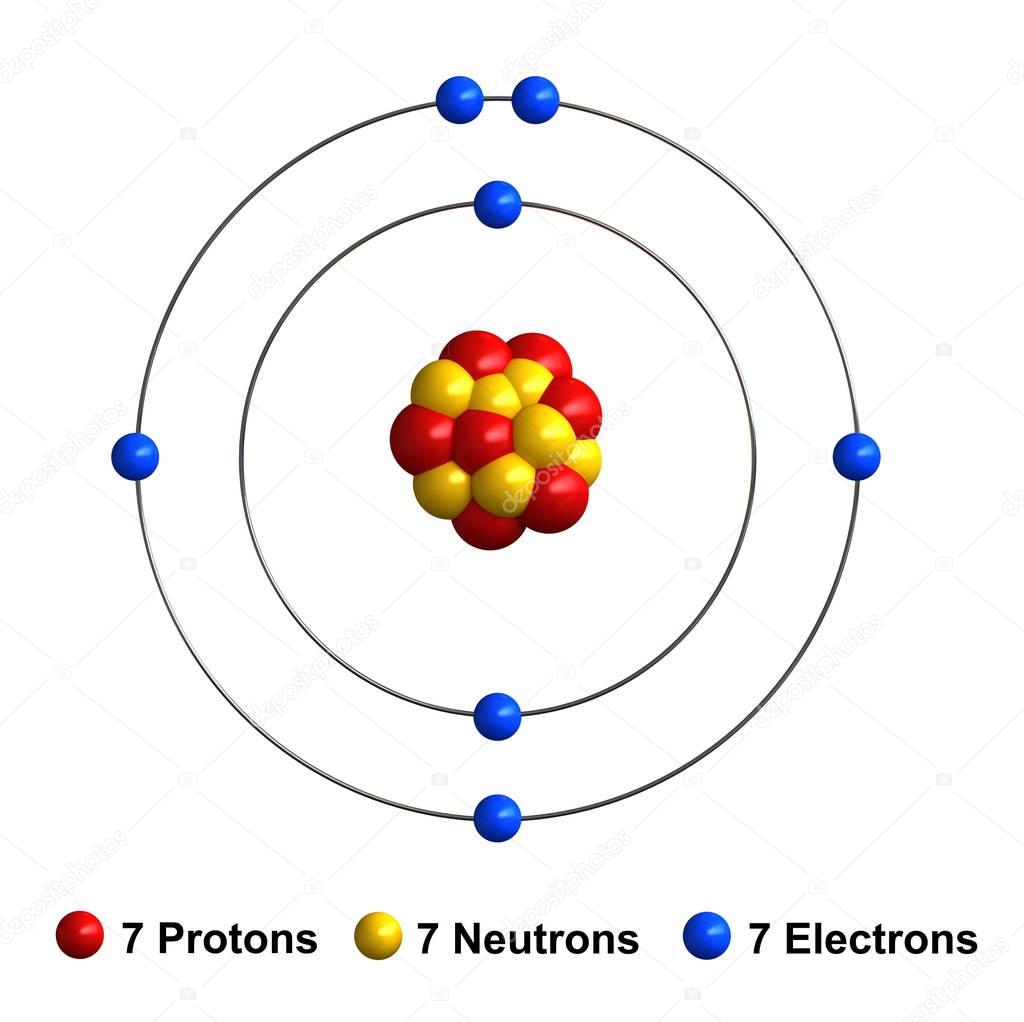
3d render of atom structure of nitrogen — Stock Photo © oorka5 136542114

Nitrogen Facts, Symbol, Discovery, Properties, Uses

Nitrogen Wikipedia

3d render of atom structure of nitrogen isolated over white background
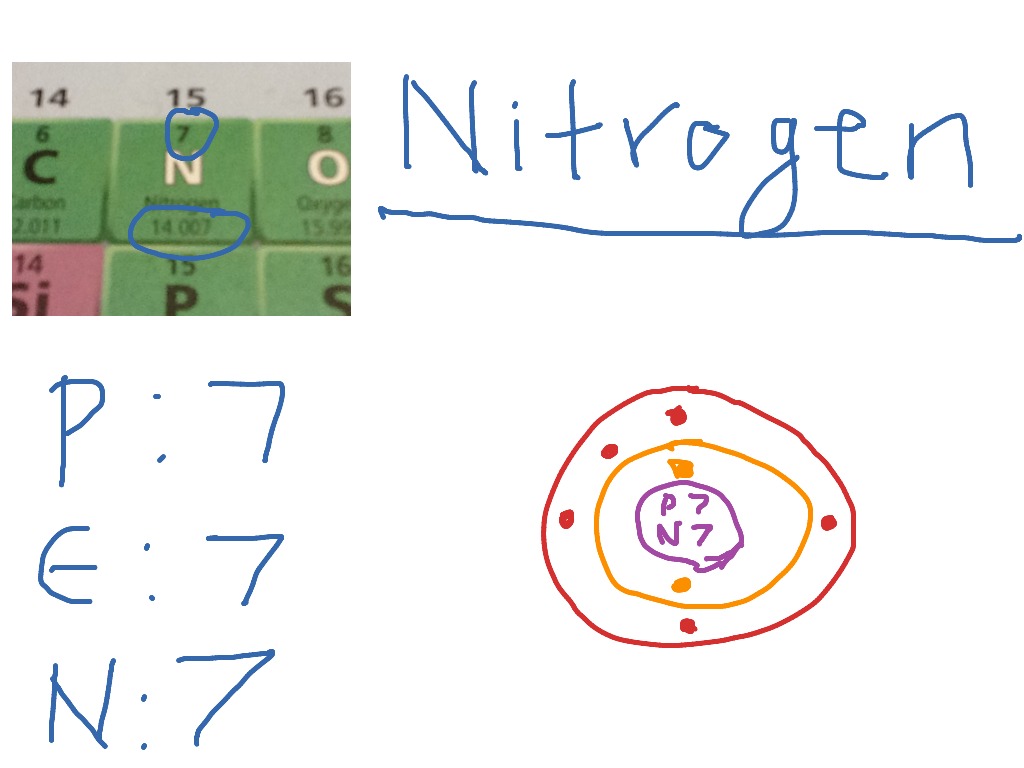
How to draw a Nitrogen Atom Science ShowMe

Bohr model Description & Development Britannica
Web How To Calculate The Formal Charge On Nitrogen.
Therefore, We Have Attained Our Most Perfect Lewis Structure Diagram.
One Lone Pair And Three Unpaired Electrons.
Draw The Lewis Diagram As Below:
Related Post: