Draw The Electron Configuration For A Neutral Atom Of Beryllium.
Draw The Electron Configuration For A Neutral Atom Of Beryllium. - Let 4 be its atomic number (z=4). A neutral chlorine atom has 17 electrons. We describe an electron configuration with a symbol that contains three pieces of information ( figure \(\pageindex{2}\)): The number of titanium is equal to 22 and an electronic configuration pc. Web the electron configuration for a neutral answer atom of beryllium is be. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): This problem has been solved! Web beryllium is the fourth element with a total of 4 electrons. Web draw the electron configuration for a neutral atom of beryllium. Now, for the electron configuration of beryllium, the first 2 electrons will go in 1s orbital since s subshell can hold a maximum of 2 electrons. Web science chemistry chemistry questions and answers o electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of beryllium. The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and Web the arrangement of electrons in the orbitals of an. Energy x 1 buy chemistry: Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The second energy level has 2s², indicating two electrons in the 2s subshell. Web beryllium is the fourth element with a total of 4 electrons. You'll get a detailed solution from a subject matter expert that. Web one cool detail that you might notice is that the electron configurations of successive elements (ordered by their periodic number) contain each other. Electron configurations describe where electrons are located around the nucleus of an atom. Web beryllium is the fourth element with a total of 4 electrons. Web draw the electron configuration for a neutral atom of beryllium.. As per the aufbau rule, the electrons will be filled into 1s orbital first then 2s,.so on. You'll get a detailed solution from a subject matter expert that helps you learn core. Energy x 1 buy chemistry: Look at the diagram carefully while i do it. Let 4 be its atomic number (z=4). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Principles and practice 3rd edition isbn: The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Web one cool detail that you might. Web a beryllium atom is a neutral atom that has 4 atomic numbers which implies it has a total of 4 electrons. As per the aufbau rule, the electrons will be filled into 1s orbital first then 2s,.so on. Since 1s can only hold two electrons the remaining 2 electrons for be go in the 2s orbital. Two electrons can. Web science chemistry draw the electron configuration for a neutral atom of beryllium. The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and Protons and neutrons, we can draw the nucleus of the beryllium atom using the postulates of the bohr atomic model. Is the same as who has to two. Now, for the electron configuration of beryllium, the first 2 electrons will go in 1s orbital since s subshell can hold a maximum of 2 electrons. Web chemistry chemistry questions and answers write the electron configuration for a neutral atom of beryllium this problem has been solved! Web the arrangement of electrons in the orbitals of an atom is called. Web one cool detail that you might notice is that the electron configurations of successive elements (ordered by their periodic number) contain each other. Web the electron configuration of beryllium is [ he] 2s 2 , if the electron arrangement is through orbitals. Energy 1 l х 5 ? Write the electron configuration for a neutral atom of beryllium show. Electron configurations describe where electrons are located around the nucleus of an atom. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. The next element is beryllium, with z = 4 and four electrons. Energy x 1 buy chemistry: You'll get a detailed solution from a. Web the electron configuration of beryllium is [ he] 2s 2 , if the electron arrangement is through orbitals. Web a beryllium atom is a neutral atom that has 4 atomic numbers which implies it has a total of 4 electrons. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) beryllium (be) electron configuration (bohr model) The atomic number of cl is 17. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When we reach boron, with z = 5 and five electrons, we must place the fifth electron in one of the 2 p orbitals. Web one cool detail that you might notice is that the electron configurations of successive elements (ordered by their periodic number) contain each other. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web rounding it up to the nearest whole number we get 9. In case the element is neutral, its atomic number will be similar to the number of electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Write the electron configuration for a neutral atom of beryllium show transcribed image text This means that the electron configuration of beryllium responds to be 1s^2 2s^2. Web march 23, 2023 by jay electron configuration chart of all elements is mentioned in the table below. We fill both the 1 s and 2 s orbitals to achieve a 1 s2 2 s2 electron configuration: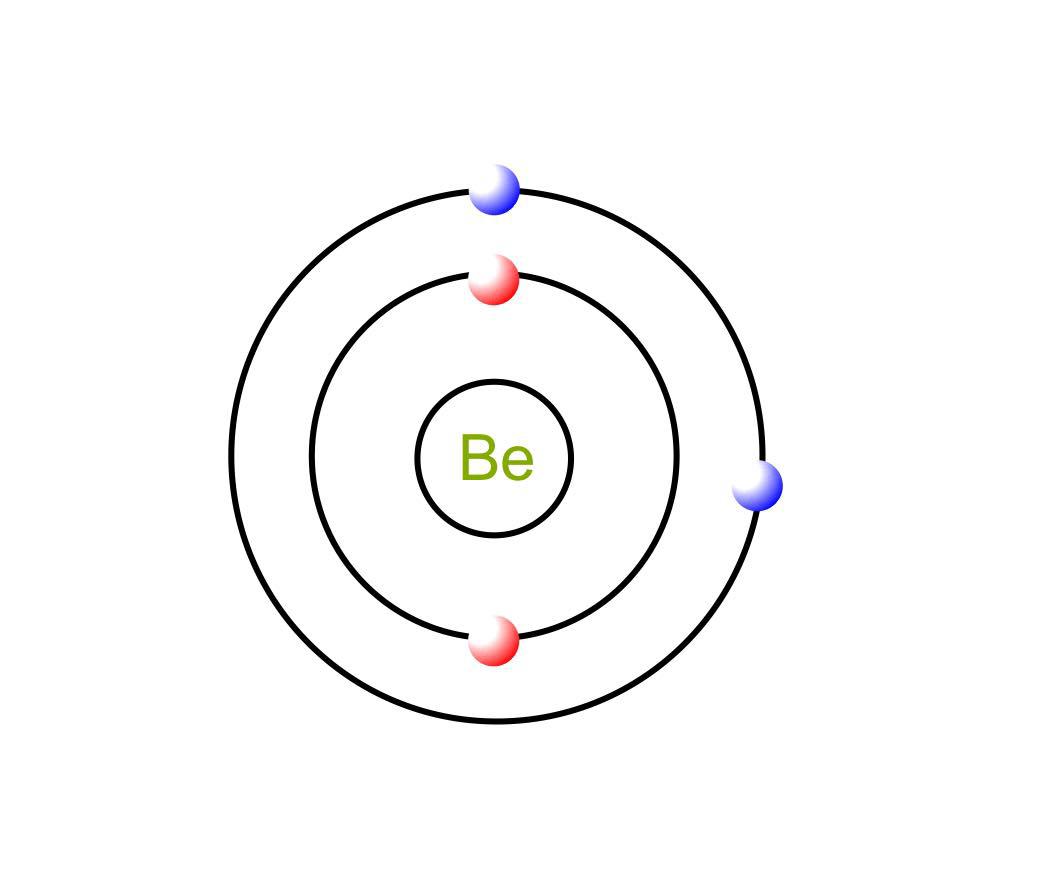
Draw a neutral Beryllium atom showing its electron shells. Quizlet

FileElectron shell 004 Beryllium.svg Wikimedia Commons Atom

How Can We Find A Beryllium Electron Configuration (Be)
Beryllium Bohr Model Diagram
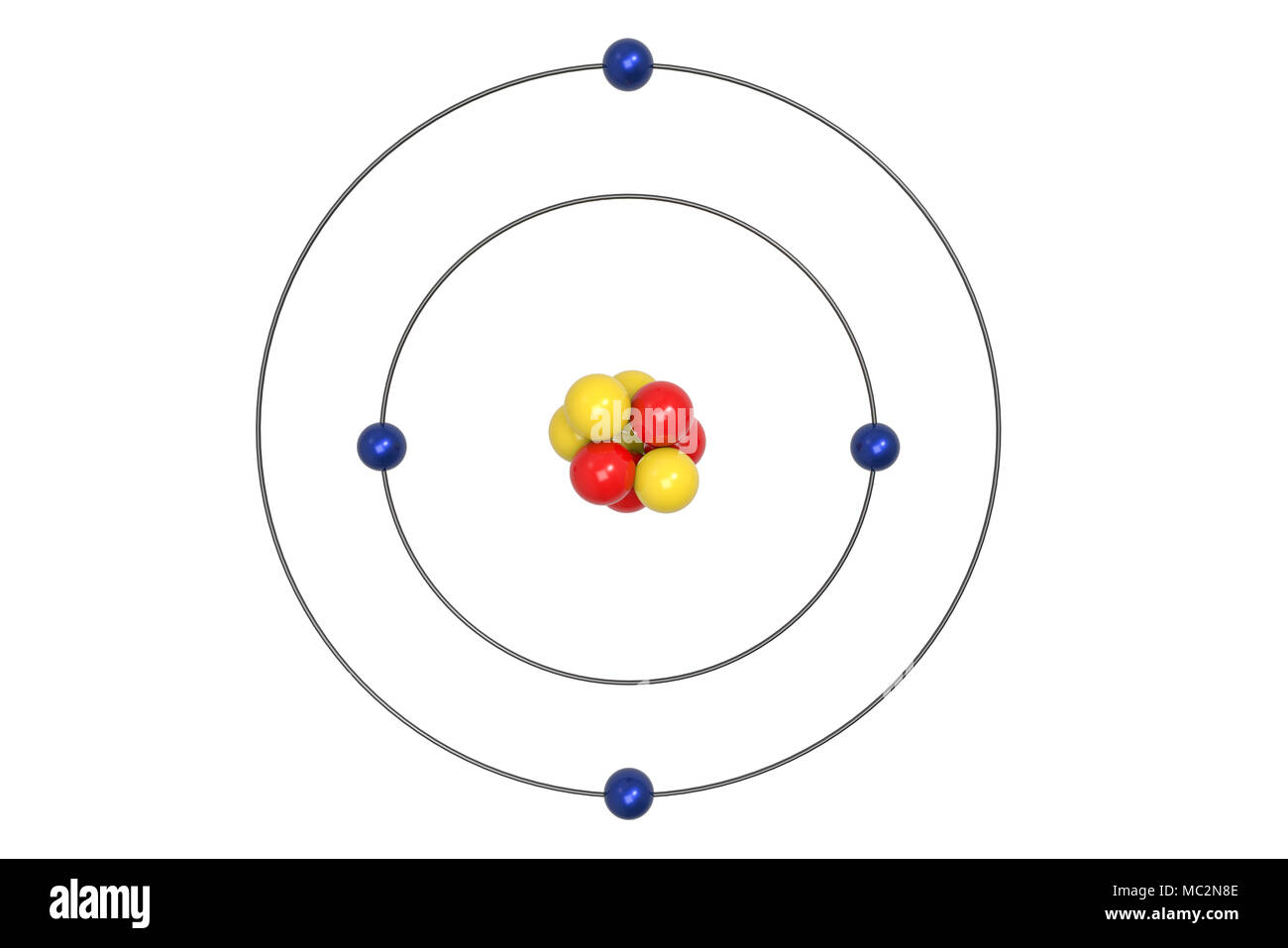
/berylliumatom-58b6028f3df78cdcd83d8f11.jpg)
Atoms Diagrams Electron Configurations of Elements

Be 2+ Electron Configuration (Beryllium Ion) YouTube

Beryllium Electron Configuration YouTube

Diagram representation of the element beryllium Vector Image
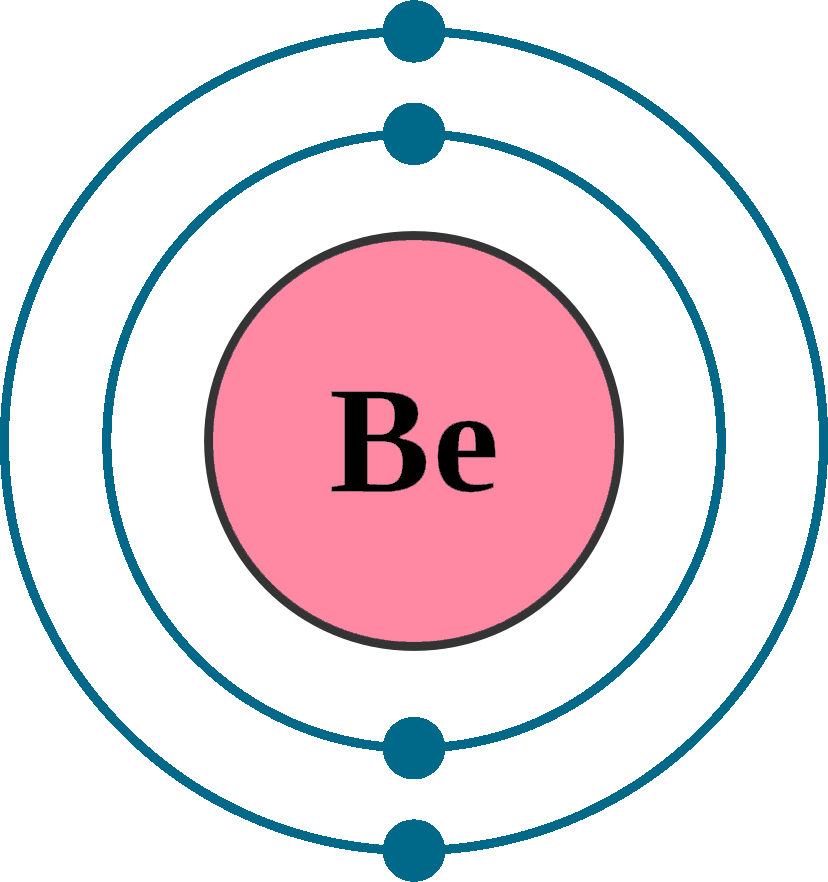
beryllium electron configuration Newton Desk

Be electronic configuration how to Write Beryllium electronic
The Next Element Is Beryllium, With Z = 4 And Four Electrons.
In Writing The Electron Configuration For Beryllium The First Two Electrons Will Go In The 1S Orbital.
Energy 1 L Х 5 ?
The Number Of The Principal Quantum Shell, N, The Letter That Designates The Orbital Type (The Subshell, L), And
Related Post: