Draw The Electron Configuration For A Neutral Atom Of Boron.
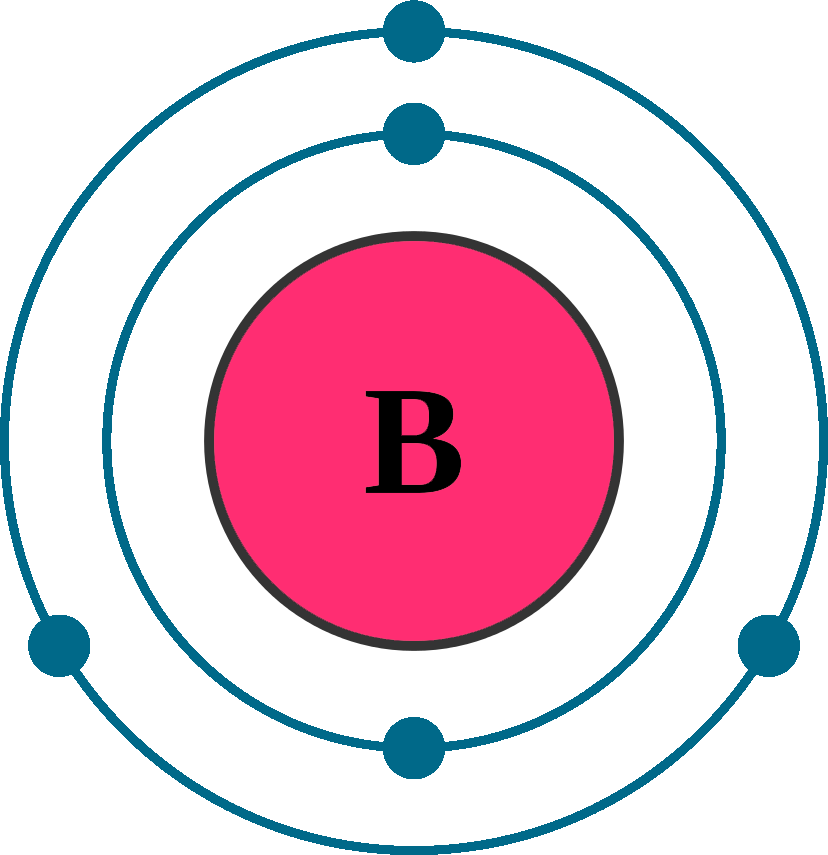
Draw The Electron Configuration For A Neutral Atom Of Boron. - A neutral helium atom, with an atomic number of 2 ( z = 2), has two electrons. Web an electrically neutral atom has the following electron configuration: Web draw the electron configuration for a neutral atom of boron. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Write the electron configuration for a neutral atom of boron. Draw the electron configuration for a neutral atom of iron. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. Energy х to this problem has been solved! The charge is equal to the number of electrons that must be gained to fill. 1s 2 2s 2 2p 1: Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Thus, it is simple to determine the charge on such a negative ion: Beryllium has two valence electrons in its 2 shell, so its electron dot diagram is like that of helium: You'll get a. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Draw the electron configuration for a neutral atom of iron. Web boron is the fifth element with a total of 5 electrons. An atom has a valence shell electron configuration of #ns^1#. Web all of the electrons in the noble gas neon (atomic number. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Web draw the electron configuration for a neutral atom of boron. Web electron configuration of boron (b) [he] 2s 2 2p 1: This problem has been solved! Web chemistry chemistry questions and answers draw the electron configuration for a neutral atom of scandium. Web electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and Electron configuration of nitrogen. Electron configuration can be done in two ways. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web boron is the fifth element with a total of 5 electrons. Draw the electron configuration for a neutral atom of boron. Energy 1 l х 5 ? At carbon, with z = 6 and six electrons, we are faced with a choice. Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of boron. Web an electrically neutral atom has the following electron configuration: Web continuing on the periodic table to the next largest atom, beryllium, with 4 electrons, the electron configuration. Web oct 1, 2016 the noble gas configuration is [he]2s^22p^1. Energy х to this problem has been solved! Its valence electron shell is 2,. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Electron configuration through orbit (bohr principle) Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. The next atom is boron. Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of boron. Energy 1 х 5 this problem has been solved!. A neutral helium atom, with an atomic number of 2 ( z = 2), has two electrons. The electron configuration of boron is [ he] 2s 2 2p 1 , if the electron arrangement is through orbitals. Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. The final ring or shell. Here’s the best way to solve it. Web an electrically neutral atom has the following electron configuration: Beryllium has two valence electrons in its 2 shell, so its electron dot diagram is like that of helium: Draw the electron configuration for a neutral atom of boron. Web oct 1, 2016 the noble gas configuration is [he]2s^22p^1. Web oct 1, 2016 the noble gas configuration is [he]2s^22p^1. Draw the electron configuration for a neutral atom of iron. Beryllium has two valence electrons in its 2 shell, so its electron dot diagram is like that of helium: Electron configuration through orbit (bohr principle) 1s 2 2s 2 2p 4: This problem has been solved! Web for each electron shell atom diagram, the element symbol is listed in the nucleus. Web draw the electron configuration for a neutral atom of boron. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Web an electrically neutral atom has the following electron configuration: The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Web its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used: Energy 1 х 5 this problem has been solved! Since 1s can only hold two electrons the next 2 electrons for b goes in the 2s orbital. Draw the electron configuration for a neutral atom of boron.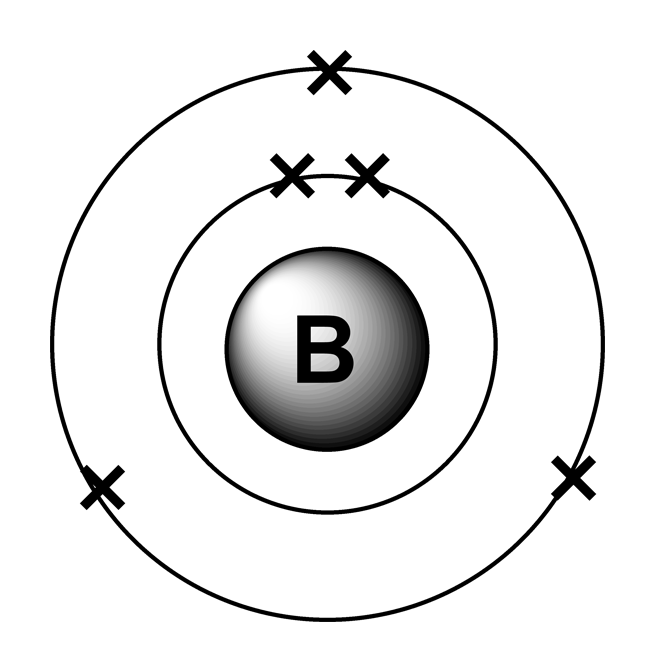
Electron arrangements
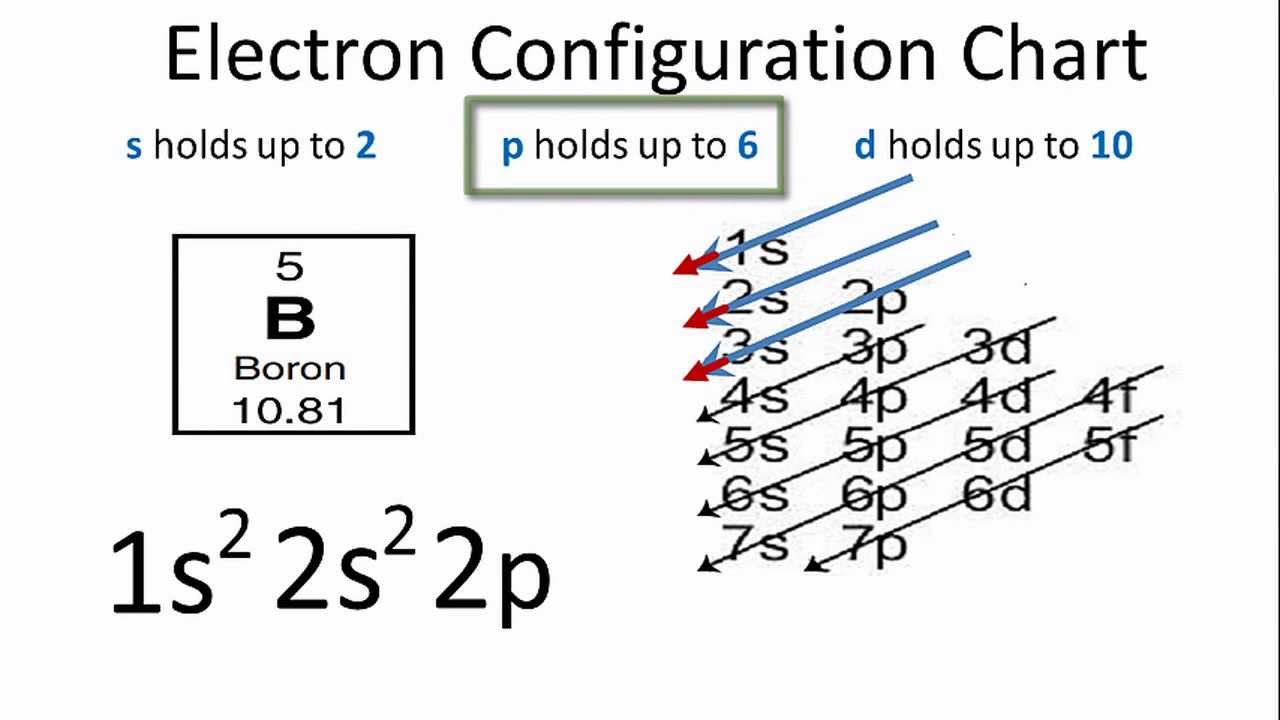
Boron Electron Configuration YouTube

Visualizing Chemistry 105 Activity 5
Boron Element With Reaction, Properties, Uses, & Price Periodic Table

Boron Electron Configuration And Full Orbital Diagram
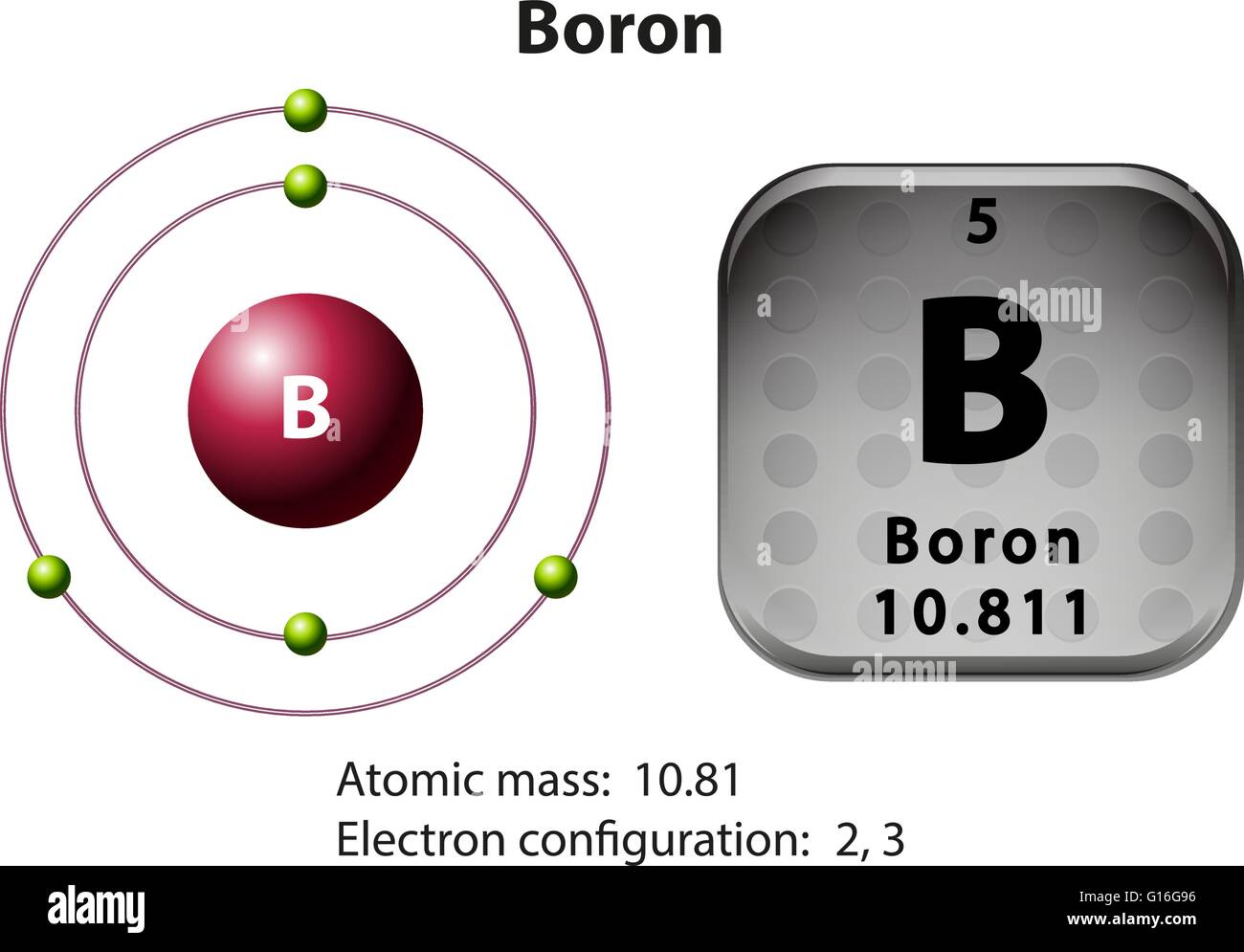
Symbol and electron diagram Boron illustration Stock Vector Image & Art
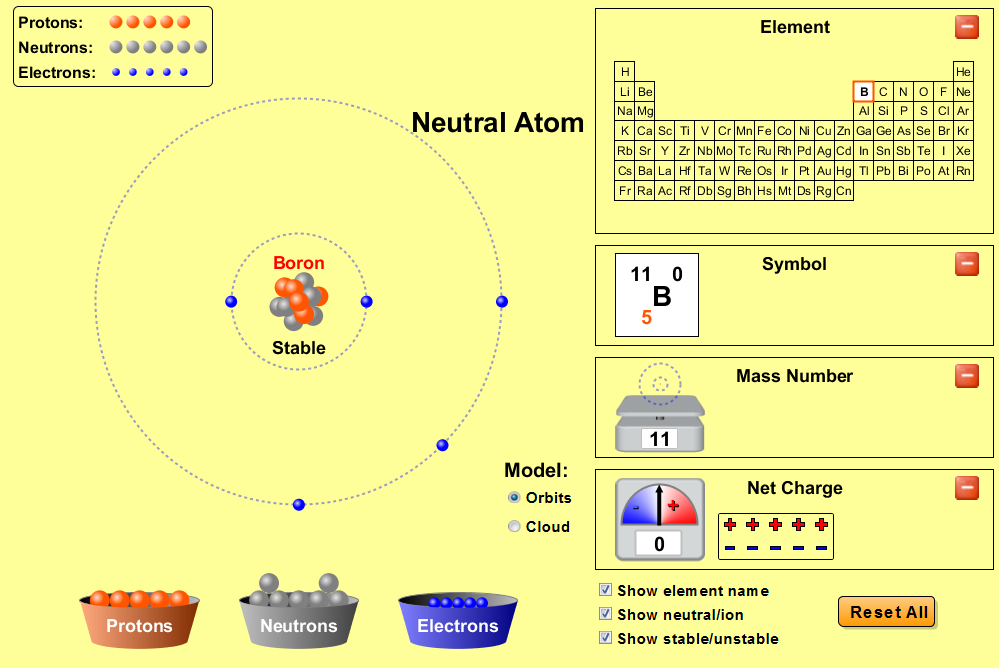
Visualizing Chemistry Activity 5
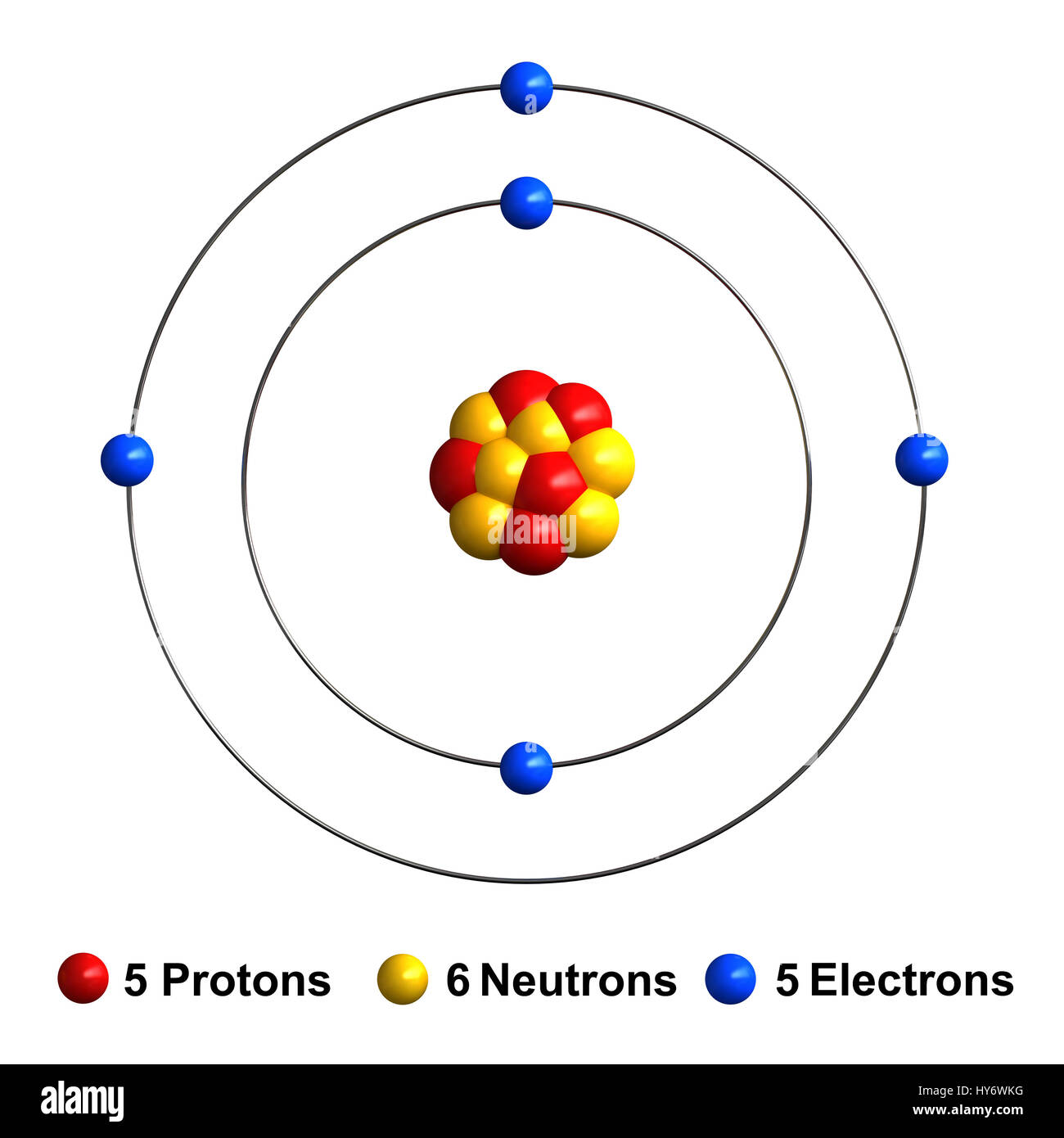
3d render of atom structure of boron isolated over white background
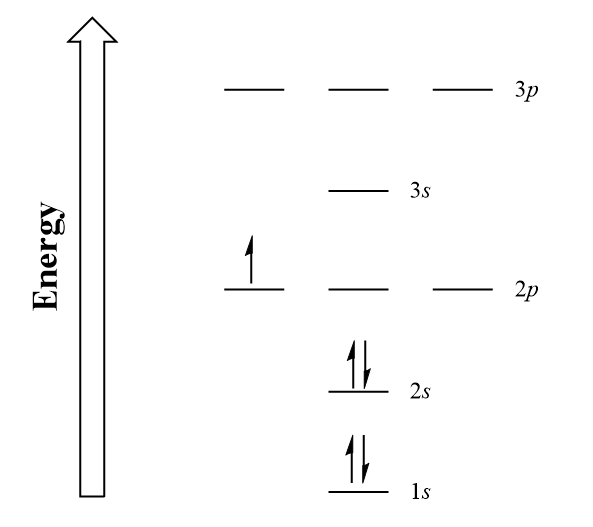
Boron_electron_configuration_energy_diagram Introductory Chemistry
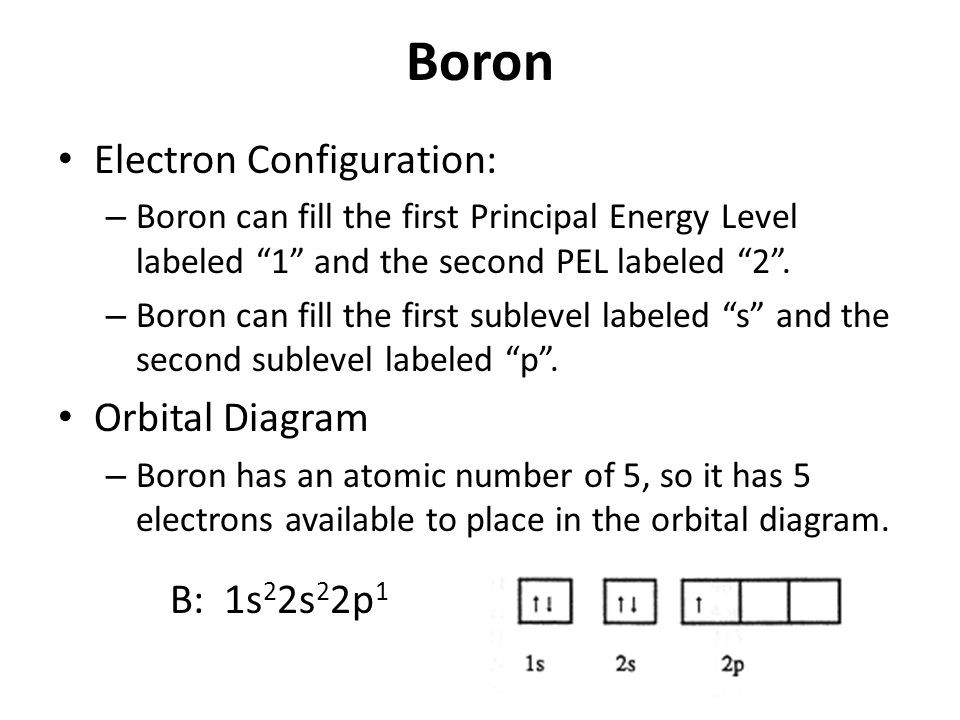
How To Find The Boron Electron Configuration (B)
Web Electron Configuration Of Boron (B) [He] 2S 2 2P 1:
Write The Electron Configuration For A Neutral Atom Of Boron.
Thus, It Is Simple To Determine The Charge On Such A Negative Ion:
Write The Electron Configuration For A Neutral Atom Of Boro.
Related Post: