Draw The Electron Configuration For A Neutral Atom Of Calcium.
Draw The Electron Configuration For A Neutral Atom Of Calcium. - Electron configuration of carbon (c) [he] 2s 2 2p 2: How many core electrons are there? Therefore, the number of electrons in neutral atom of calcium is 20. Calcium has an atomic number of 20, which means it has 20 electrons in a neutral atom. The valence electrons are found in the highest energy level of the electron configuration in the 's' and 'p' orbitals. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. 1s 2 2s 2 2p 1: What about potassium, and what about for chlorine? 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s,. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Energy х this problem has been solved! Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Web in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. Following hydrogen is the noble gas. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Electron configuration can be done in two ways. Determine whether the substance is paramagnetic or diamagnetic Following hydrogen is the noble gas helium, which has an atomic number of 2. An atom has a valence shell electron. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. Electron configuration of carbon (c) [he] 2s 2 2p 2: A neutral chlorine atom has 17 electrons. The next six electrons will go in the 2p orbital. Hence, calcium has 2. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2) how do we write the configuration for ca^ (2+)? Web for nitrogen this would be 2.5 or 2,5 and for calcium this would be 2.8.8.2 or 2,8,8,2. Put the noble gas in brackets and write the remainder of the electron configuration. Electron configuration can be done in two ways. Web draw the electron. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2) how do we write the configuration for ca^ (2+)? Ignore the core electrons and focus on the valence electrons only. How many core electrons are there? Sodium (na) has atomic number 11, hence, 11 electrons. Locate the noble gas element in the period above the element of interest. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. This means the first shell (1s) has 2. Hence, calcium has 2 valence electrons. Web for nitrogen this would be 2.5 or 2,5 and for calcium this would be 2.8.8.2 or. Ignore the core electrons and focus on the valence electrons only. Since 1s can only hold two electrons the next 2 electrons for calcium go in the 2s orbital. Simply use this information to obtain its electronic configuration. Web locate the atom on the periodic table. The valence electrons are found in the highest energy level of the electron configuration. Simply use this information to obtain its electronic configuration. Following hydrogen is the noble gas helium, which has an atomic number of 2. Hence, calcium has 2 valence electrons. How many core electrons are there? Ignore the core electrons and focus on the valence electrons only. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. Web an electrically neutral atom has the following electron configuration: Electron configuration of carbon (c) [he] 2s 2 2p 2: Lliruns in aiums and molecules drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom. When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the calcium atom. The number of shells shows which period, or row, it’s in and the number of electrons in the outer shell shows which group it’s in. The 4th energy level (row), 's' orbital block, 2nd group (column). Web electron configuration of beryllium. Electron configuration can be done in two ways. Web examine the electron configuration of neutral calcium atom (exercise \(\pageindex{2}\)), 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2, and write the abbreviated notation. Calcium has an atomic number of 20, which means it has 20 electrons in a neutral atom. Web in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. Electron configuration of carbon (c) [he] 2s 2 2p 2: Lliruns in aiums and molecules drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of calcium. Web an electrically neutral atom has the following electron configuration: The helium atom contains two protons and two electrons. The arrangement of an element’s electrons tells you where it is on the periodic table. And the abridged form is [ar]4s^2. Simply use this information to obtain its electronic configuration. Continue the electron configuration from the noble gas until you reach the element of interest. The valence electrons are found in the highest energy level of the electron configuration in the 's' and 'p' orbitals. Determine whether the substance is paramagnetic or diamagnetic You'll get a detailed solution from a subject matter expert that helps you learn core concepts.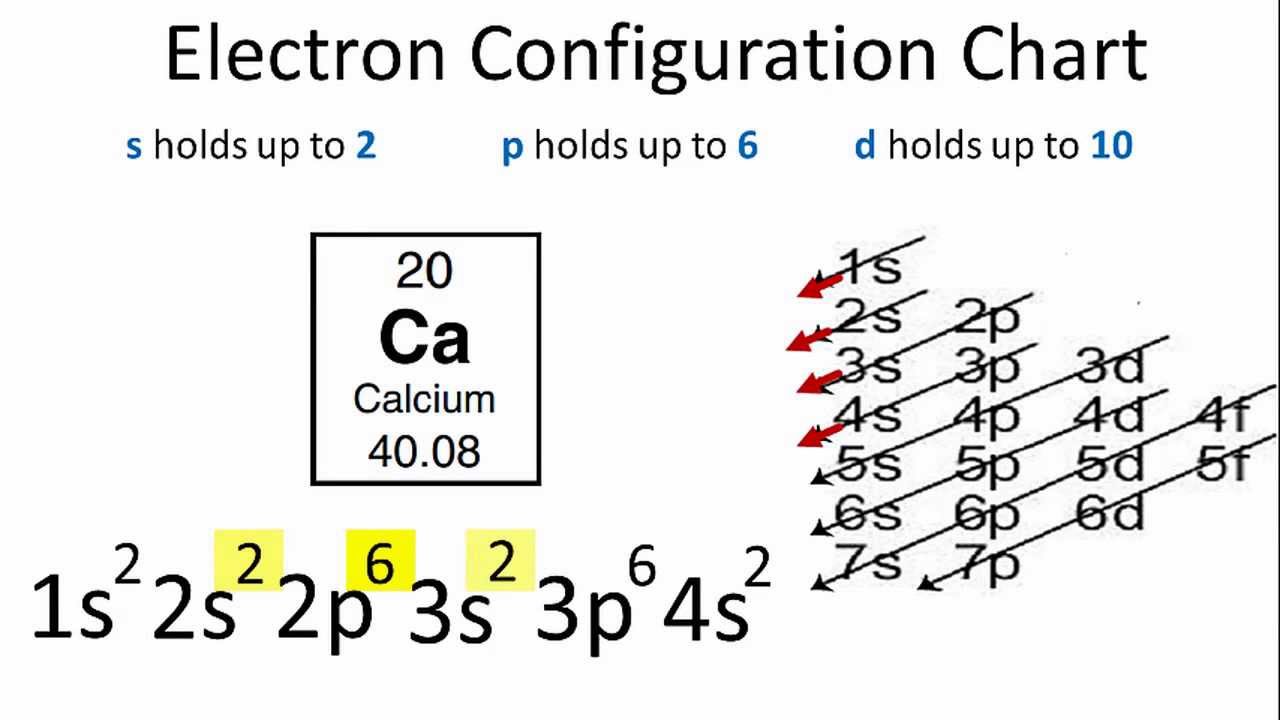
Calcium Electron Configuration (Ca) with Orbital Diagram

Bohr Diagram For Calcium

Draw The Electron Configuration For A Neutral Atom, HD Png Download

Calcium Facts

Calcium, atomic structure Stock Image C018/3701 Science Photo Library
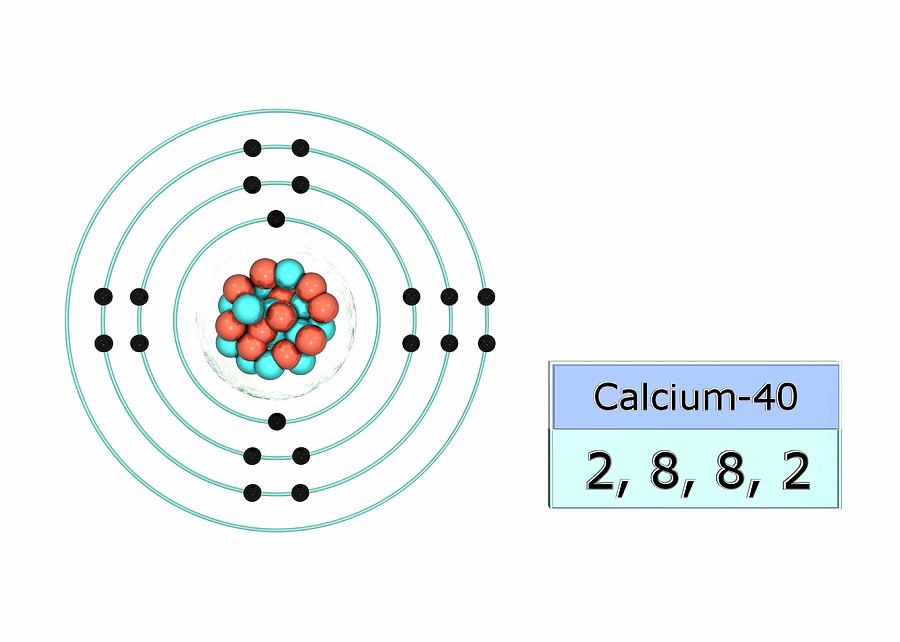
Calcium Electron Dot Diagram Photos Cantik
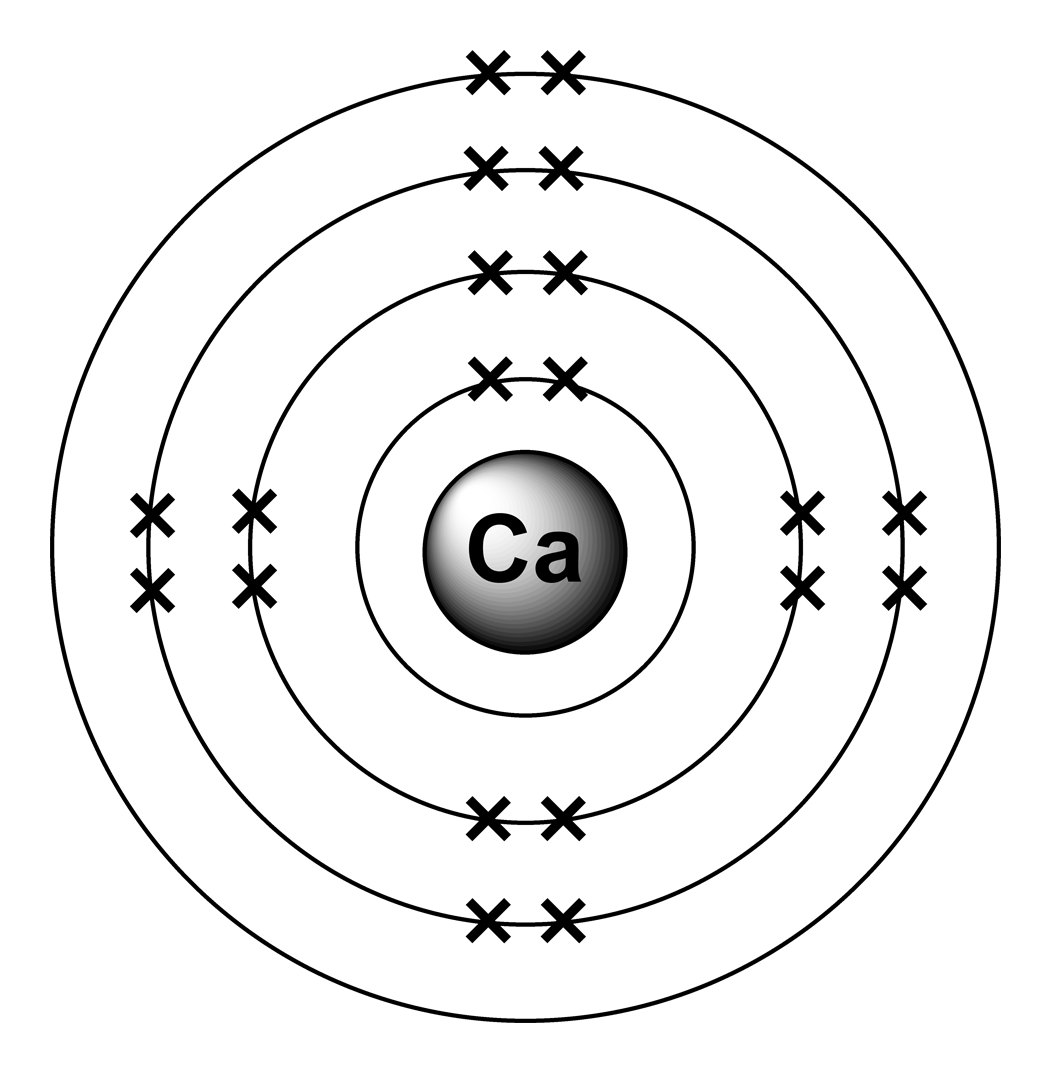
Electron arrangements

Calcium (Ca) electron configuration and orbital diagram (2023)

How Many Valence Electrons are in Calcium Periodic Table
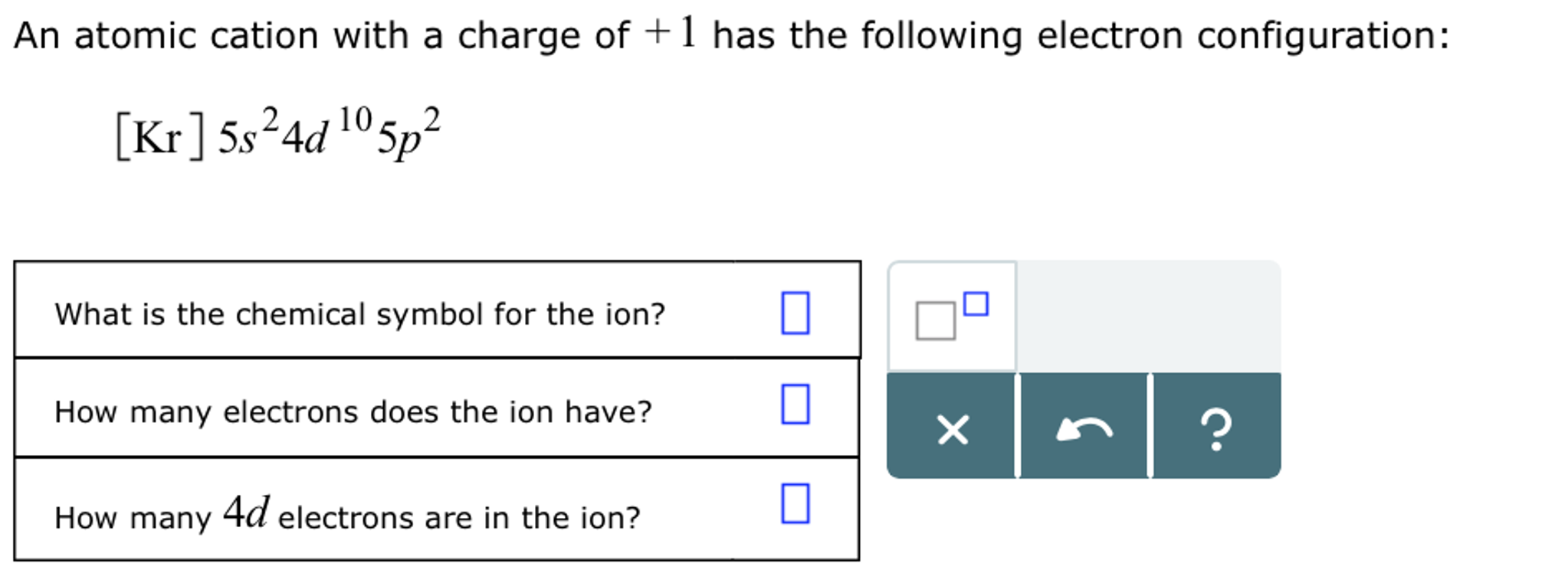
(Get Answer) Draw The Electron Configuration For A Neutral Atom Of
Web In Order To Write The Calcium Electron Configuration We First Need To Know The Number Of Electrons For The Ca Atom (There Are 20 Electrons).
Web Electrons And Electron Configuration.
Web The Electron Configuration Of Calcium Is [ Ar] 4S 2 , If The Electron Arrangement Is Through Orbitals.
1S 2 2S 2 2P 3:
Related Post: