Draw The Electron Configuration For A Neutral Atom Of Iron.
Draw The Electron Configuration For A Neutral Atom Of Iron. - And so first, let's just think about the electron configuration of the simplest element. Web an electrically neutral atom has the following electron configuration: Web and to help us with that, we will look at a periodic table of elements. Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? Because there are no unpaired electrons, zn atoms are diamagnetic. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Thus, it is simple to determine the charge on such a negative ion: Electron configuration can be done in two ways. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Web chemistry chemistry questions and answers draw the electron configuration for a neutral atom of iron. The number of the principal quantum shell, n, the. Thus, it is simple to determine the charge on such a negative ion: Once we have the configuration for fe, the ions are simple. For zn atoms, the electron configuration is 4s 2 3d 10. Locate the noble gas element in the period above the element of interest. The electron configuration of iron is [ ar] 3d 6 4s 2. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. This is sometimes called the bohr, or the ‘solar system’, model. Note that the total number of electrons in the neutral atom adds up to the atomic number, so 2+2+6+2+6 = 18, which is the atomic number of ar. Electrons are represented by dots or. Draw the electron configuration for a neutral atom of iron. There are no unpaired electrons. The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and Thus, it is simple to determine the charge on such a negative ion: Web in order to write the electron configuration for iron (fe) we first. The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating. Put the noble gas. Note that the total number of electrons in the neutral atom adds up to the atomic number, so 2+2+6+2+6 = 18, which is the atomic number of ar. Web the electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. 1s 2 2s 2 2p 3: Web the arrangement of. Web electron configuration of nitrogen (n) [he] 2s 2 2p 3: What is the name of this atom? Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Web in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? 1s 2 2s 2 2p 3: Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Because there are no unpaired electrons, zn atoms are diamagnetic. Web the arrangement of. This is sometimes called the bohr, or the ‘solar system’, model. Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? Web let's begin this section with the orbital box (or the orbital representation diagram) for a neutral atom. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. You'll get a detailed solution from a. Once we have the configuration for fe, the ions are simple. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Electron configuration can be done in two ways. If we're talking about a neutral hydrogen atom, a neutral hydrogen atom, it has an atomic number of one which tells us. What is the name of this atom? Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. 1s 2 2s 2 2p 6: 1s 2 2s 2 2p 4: Energy this problem has been solved! The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. Step 1/2 the atomic number of iron is. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. Once we have the configuration for fe, the ions are simple. Draw the electron configuration for a neutral atom of iron. Draw a lewis electron dot diagram for an atom or a monatomic ion. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Web in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Electron configuration of neon (ne) [he] 2s 2 2p 6: Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle.:max_bytes(150000):strip_icc()/Iron-58b602243df78cdcd83d3d5a.jpg)
Atoms Diagrams Electron Configurations of Elements

【5 Steps】Electron Configuration of Iron(Fe) Electron configuration

Iron Protons Neutrons Electrons Electron Configuration

Get the Detailed Periodic table (With Electron Configuration)

Flashcard iron with atomic mass Royalty Free Vector Image
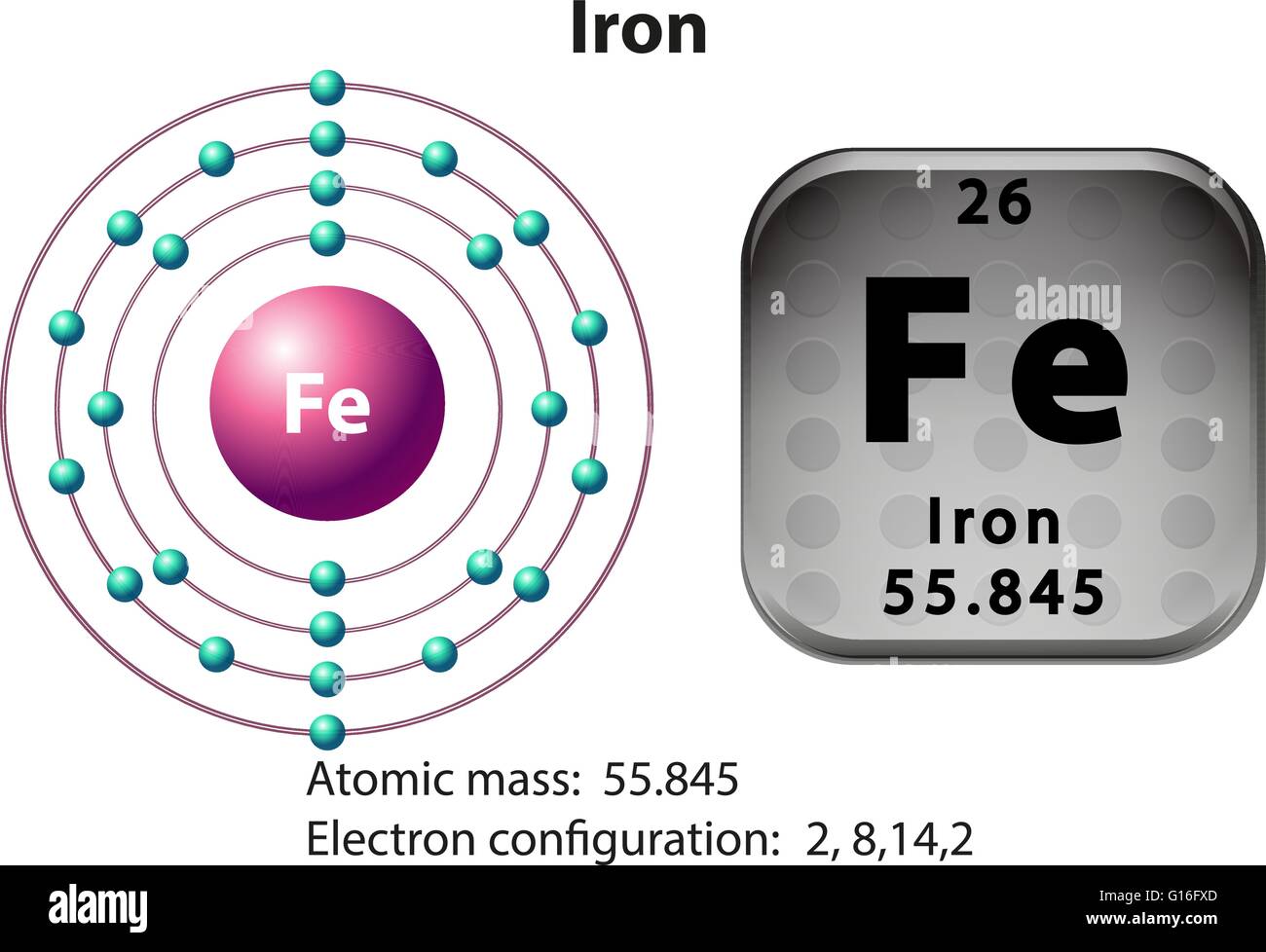
Symbol and electron diagram for Iron illustration Stock Vector Image
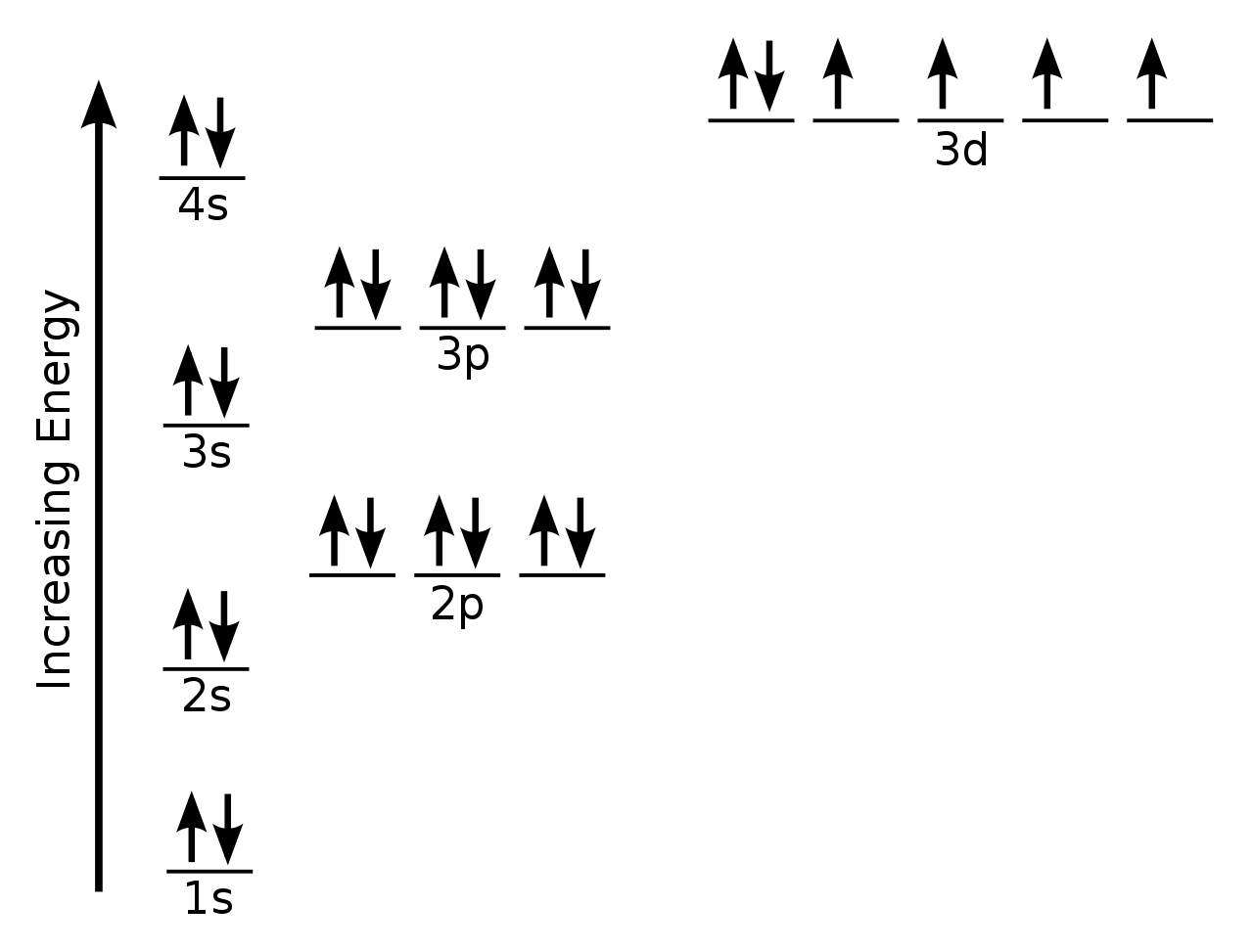
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB)

Draw the electron configuration for a neutral atom of iron. Quizlet

Iron electronic configuration How to Write Iron electronic
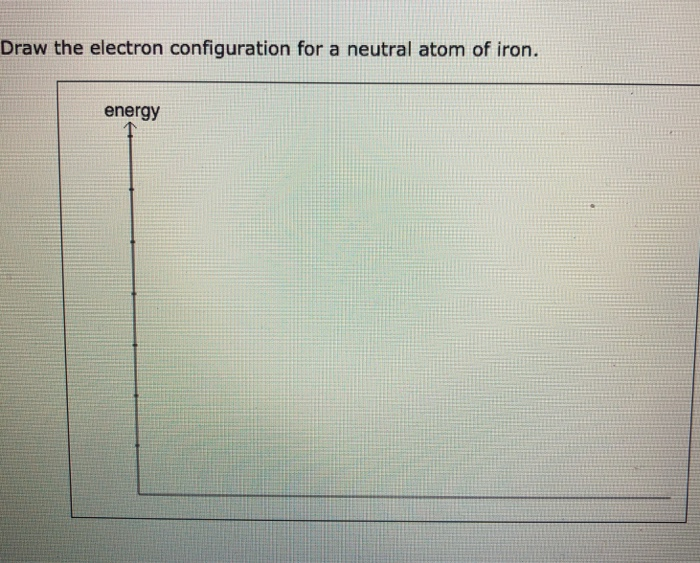
Solved Draw the electron configuration for a neutral atom of
We Add Electrons To Fill The Outermost Orbital That Is Occupied, And Then Add More Electrons To The Next Higher Orbital.
Locate The Noble Gas Element In The Period Above The Element Of Interest.
Web An Electron Configuration Diagram Is A Model That Depicts The Position Of Electrons As They Orbit The Nucleus Of An Atom.
Web Chemistry Chemistry Questions And Answers Draw The Electron Configuration For A Neutral Atom Of Iron.
Related Post: