Draw The Lewis Dot Diagram For A Cation
Draw The Lewis Dot Diagram For A Cation - Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web the lewis structure of an ionic compound represents the distribution of electrons in the compound. Web draw lewis structures for ionic compounds. Web the lewis dot structure for the hydroxide ion. Web learning objectives draw a lewis electron dot diagram for an atom or a monatomic ion. Web chemistry chemistry questions and answers draw the lewis dot diagram for a cl2+ cation. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web write the lewis structure for each molecule or ion. Ch3oo (unpaired electron on terminal oxygen) 1 shows the lewis symbols for the elements of the third period of the periodic table. Web the lewis dot structure for the hydroxide ion. Also, helium is shown in group 8a, but it only has two valence electrons. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Having lost its two original valence. Web now most people will represent this dot structure by putting brackets here. Web draw lewis structures for ionic compounds. Draw the lewis dot diagram for a cl2+ cation. Having lost its two original valence electrons, the lewis electron dot diagram is just ca 2+. Draw the lewis dot diagram for a cl2+ cation. Web chemistry chemistry questions and answers draw the lewis dot diagram for a cl2+ cation. Dots around o indicate three lone pairs, and the line between h and o represents a covalent bond containing two shared electrons. The astute reader may have noticed something: The following is a general procedure for drawing lewis structures. Many of the. Include resonance structures if necessary and. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Shared pairs. Web now most people will represent this dot structure by putting brackets here. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: The number of dots equals. Web. Web chemistry chemistry questions and answers draw the lewis dot diagram for a cl2+ cation. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. The immune system then attacks the cells and destroys them, weakening the. The following is a general procedure for drawing lewis structures. Web we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Many of the ions that form have eight electrons in their valence shell. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms.. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web free radicals may also attack molecules on the surfaces of cells, making them appear foreign to the body's immune system. Web write the lewis structure for each molecule or ion. A lewis symbol consists. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Include resonance structures if necessary and. Figure 7.9 shows the lewis symbols for the elements of the third period of the periodic table. Web we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Draw the lewis dot diagram for a. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web what is the lewis electron dot diagram for each ion? The lewis structure of an ionic compound is not necessarily a single molecule but rather a repeating pattern of cations and anions arranged in a. Many of. To write lewis dot structure of an atom, we write the symbol of the atom and then draw dots one for e. The astute reader may have noticed something: And putting a positive charge outside of it. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Ch3oo (unpaired electron on terminal oxygen) The lewis structure of an ionic compound is not necessarily a single molecule but rather a repeating pattern of cations and anions arranged in a. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Also, helium is shown in group 8a, but it only has two valence electrons. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Draw the lewis dot structure for a c2+ cation. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. A lewis electron dot diagram
How to Draw a Lewis Structure
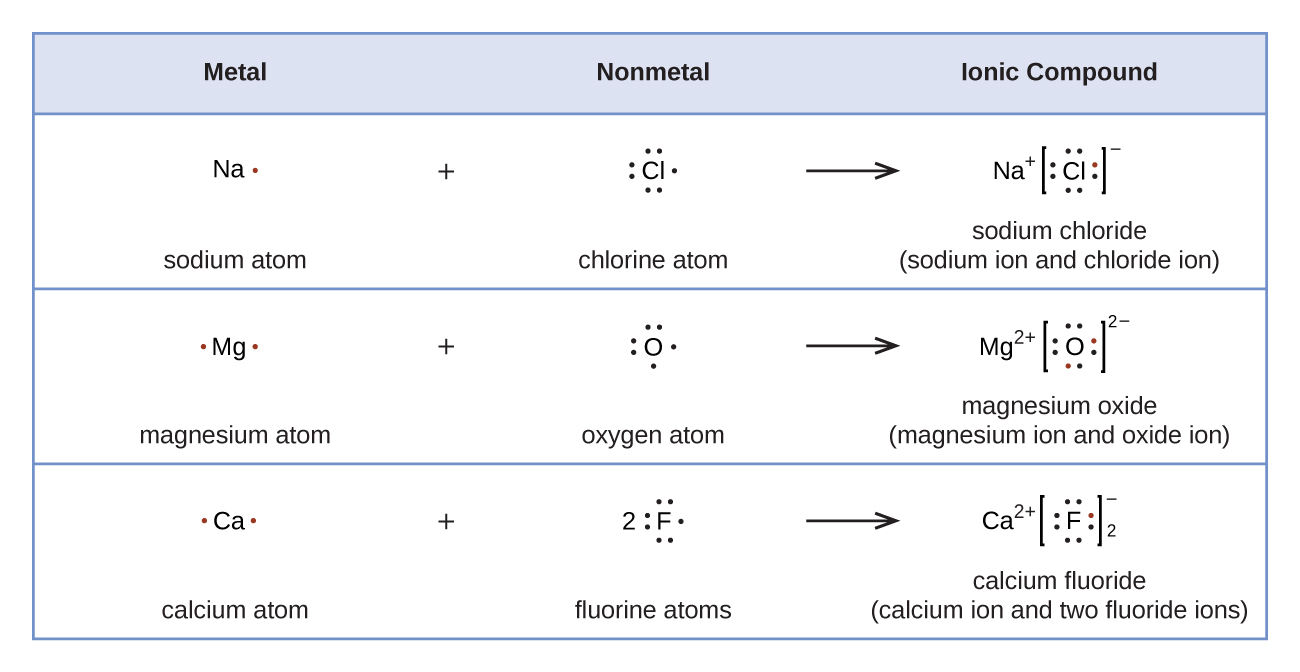
3.2 Lewis Dot Diagrams Chemistry LibreTexts

Lewis Electron Dot Structure Calculator

Lewis Dot Structure Definition, Examples, and Drawing

Comment dessiner une représentation de Lewis Wiki Chimie

Lewis Dot Structures of Atoms and Ions YouTube

3 Ways to Draw Lewis Dot Structures wikiHow

Lewis Structures Made Easy Examples and Tricks for Drawing Lewis Dot

How to Draw Lewis Dot Structure

Lewis Structure Types
Having Lost Its Two Original Valence Electrons, The Lewis Electron Dot Diagram Is Just Ca 2+.
Web How To Draw Lewis Structures.
Dots Around O Indicate Three Lone Pairs, And The Line Between H And O Represents A Covalent Bond Containing Two Shared Electrons.
The Following Is A General Procedure For Drawing Lewis Structures.
Related Post: