Draw The Lewis Structure For Bcl3
Draw The Lewis Structure For Bcl3 - Bcl3 draw the lewis dot structure for bcl3. View the full answer answer unlock unlock previous question next question transcribed image text: For the bcl3 structure use the periodic table to find the total number of valence electrons. Web drawing the lewis structure for bcl 3. Breaking the octet rule ; Web drawing lewis structures for molecules with one central atom: There are three lone pairs on each chlorine atom, and the boron atom does not have any lone pair. It is a colorless inorganic compound that has a pungent odor and appears as fumes in air. Web bcl3 lewis structure and molecular geometry. In order to draw the lewis. Each of your answers should consist of either an element symbol (do not write out the entire element name to avoid spelling errors) or a whole number value. Web steps here’s how you can easily draw the bcl 3 lewis structure step by step: Use your structure to answer the questions below. Boron (b) doesn't need 8 valence electrons to. Boron trichloride (bcl3) is a chemical compound that consists of one boron atom and three chlorine atoms. To sketch the bcl3 lewis. There are 26 valence electrons. Web the lewis structure of bcl3 contains three single bonds, with boron in the center, and three chlorines on either side. For the bcl3 structure use the periodic table to find the total. Using formal charges to determine how many bonds to make, a different perspective. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web 5 steps to draw the lewis structure of bcl3 step #1: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw the lewis structure. Boron, b, is an exception to the octet rule. In order to draw the lewis. Web step 1 the lewis structure of bcl a 3. The bcl 3 lewis structure is similar to bf 3 and bbr 3 since f and br are in group 7 and have 7 valence electrons. Web how to draw bcl3 lewis structure? Web how to draw the bcl3 lewis structure. Boron (b) doesn't need 8 valence electrons to have an octet (boron often only needs 6). In order to draw the lewis. Web draw the lewis structure and determine the molecular geometry for the following compounds: Web draw (on paper) a lewis structure for bcl3 and answer the following questions based on. Web draw the lewis structure and determine the molecular geometry for the following compounds: Each of your answers should consist of either an element symbol (do not write out the entire element name to avoid spelling errors) or a whole number value. There are 26 valence electrons. Calculate the total number of valence electrons. Breaking the octet rule ; Boron trichloride (bcl3) is a chemical compound that consists of one boron atom and three chlorine atoms. Valance electron in bcl 3 = 3+ (7x3). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Using formal charges to determine how many bonds to make, a different perspective. This is free chemistry help for. Web step 1 the lewis structure of bcl a 3. Web bcl3 lewis structure and molecular geometry. Web to find out the lewis dot structure we first find out the valence electrons and then start arranging atoms to show bond formation. (can be more then one) a) dispersion. Web drawing lewis structures for molecules with one central atom: Part b determine molecular geometry of bcl3. Web drawing the lewis structure for bcl 3. Web how to draw the bcl3 lewis structure. Follow the steps below to draw the lewis structure of bcl3. Web to find out the lewis dot structure we first find out the valence electrons and then start arranging atoms to show bond formation. Draw the molecule by placing atoms on the grid and connecting them with bonds. First, lets find the how many valence electrons chlorate has: Next lets draw the basic framework of the molecule: Web step 1 the lewis structure of bcl a 3. Web a video explanation of how to draw the lewis dot structure for boron trichloride, along with. Each of your answers should consist of either an element symbol (do not write out the entire element name to avoid spelling errors) or a whole number value. Web written by priyanka in science a trihalide of boron, bcl 3 consists of a single boron atom and three atoms of chlorine. Web step 1 the lewis structure of bcl a 3. The bcl 3 lewis structure is similar to bf 3 and bbr 3 since f and br are in group 7 and have 7 valence electrons. This is free chemistry help for you. Web draw the lewis structure for bcl 3. The number of lone pairs the number of single bonds = the number of double bonds = 2. The number of lone pairs = ___ the this problem has been solved! This video tutorial will explain how to draw the lewis dot structure and molecular geometry for boron. Boron trichloride (bcl3) is a chemical compound that consists of one boron atom and three chlorine atoms. Bcl3 draw the lewis dot structure for bcl3. Boron, b, is an exception to the octet rule. Web draw (on paper) a lewis structure for bcl3 and answer the following questions based on your drawing. (can be more then one) a) dispersion. Web how to draw bcl3 lewis structure? Breaking the octet rule ;
draw the lewis structure for bcl3 in the marvin window below and then
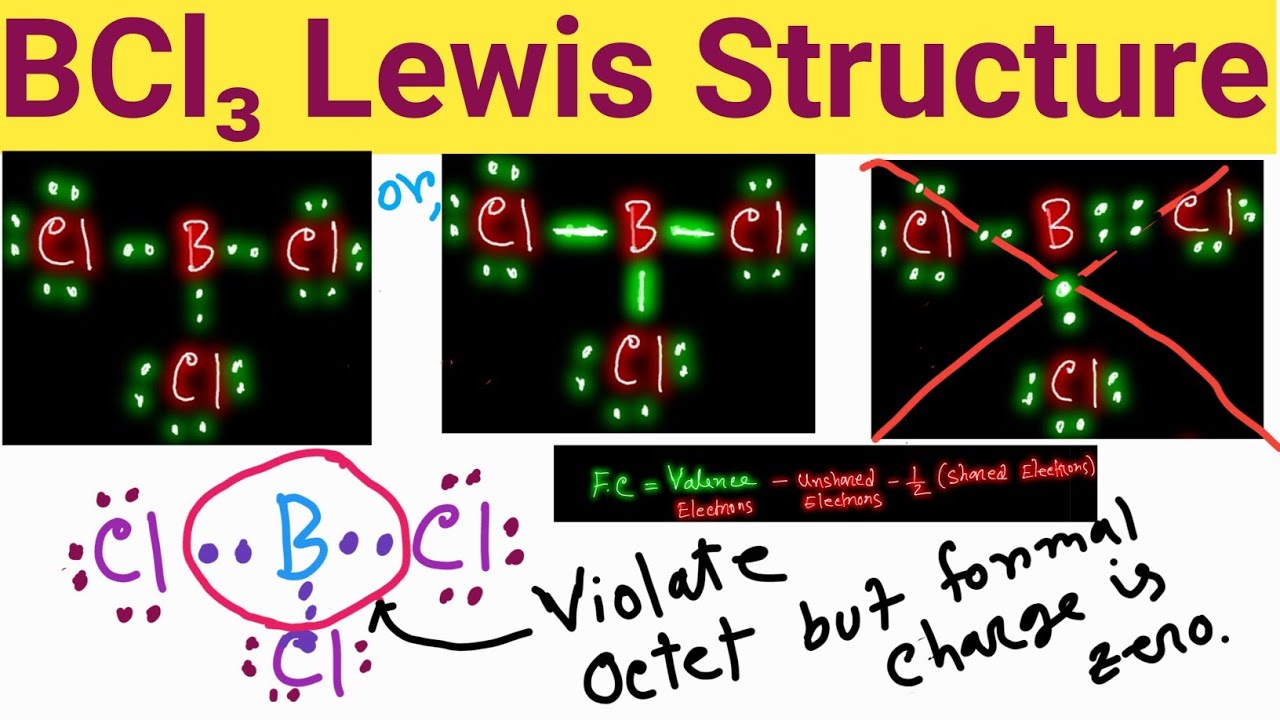
BCl3 Lewis Structure Lewis Dot Structure for BCl3 Boron Trichloride

BCl3 Lewis Structure YouTube

Lewis dot structure of BCl3 Boron trichloride lewis structure

BCl3 Lewis Structure, Molecular Geometry, and Hybridization

Draw a Lewis structure for BCl3 and answer the following questions
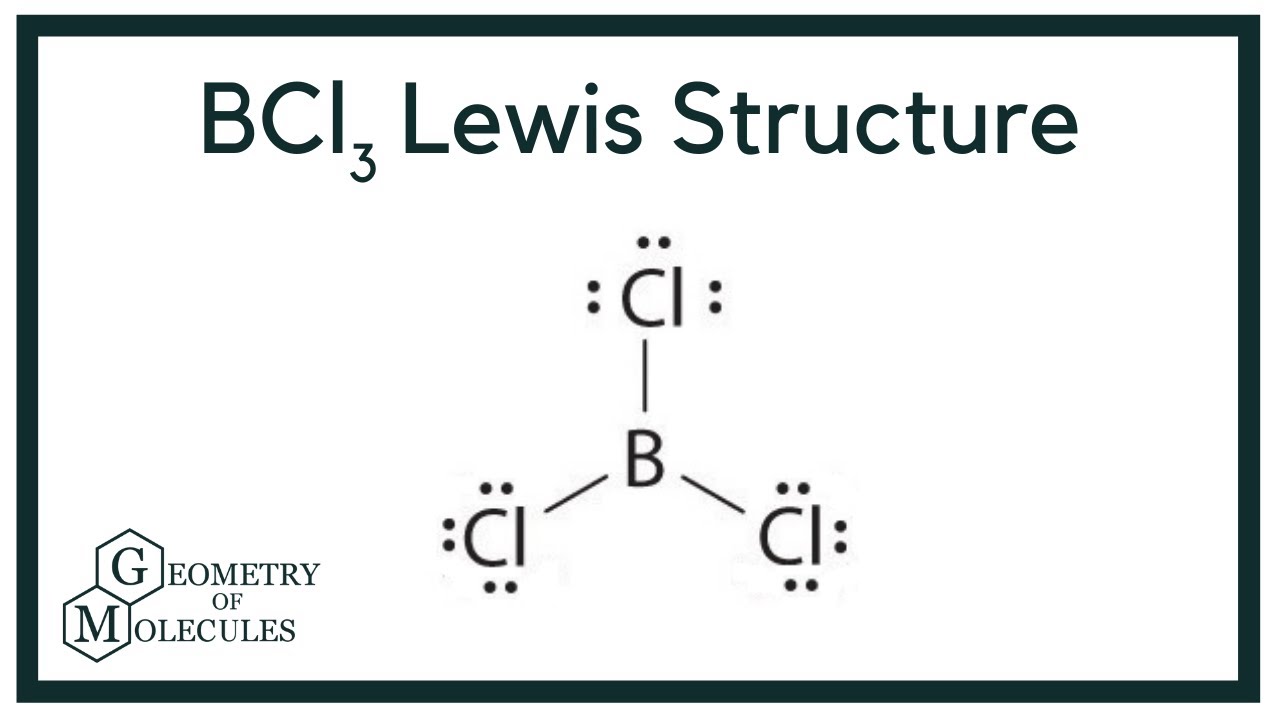
BCl3 Lewis Structure (Boron Trichloride) YouTube
Bcl3 Lewis Dot Structure Draw The Lewis Structure With The Lowest
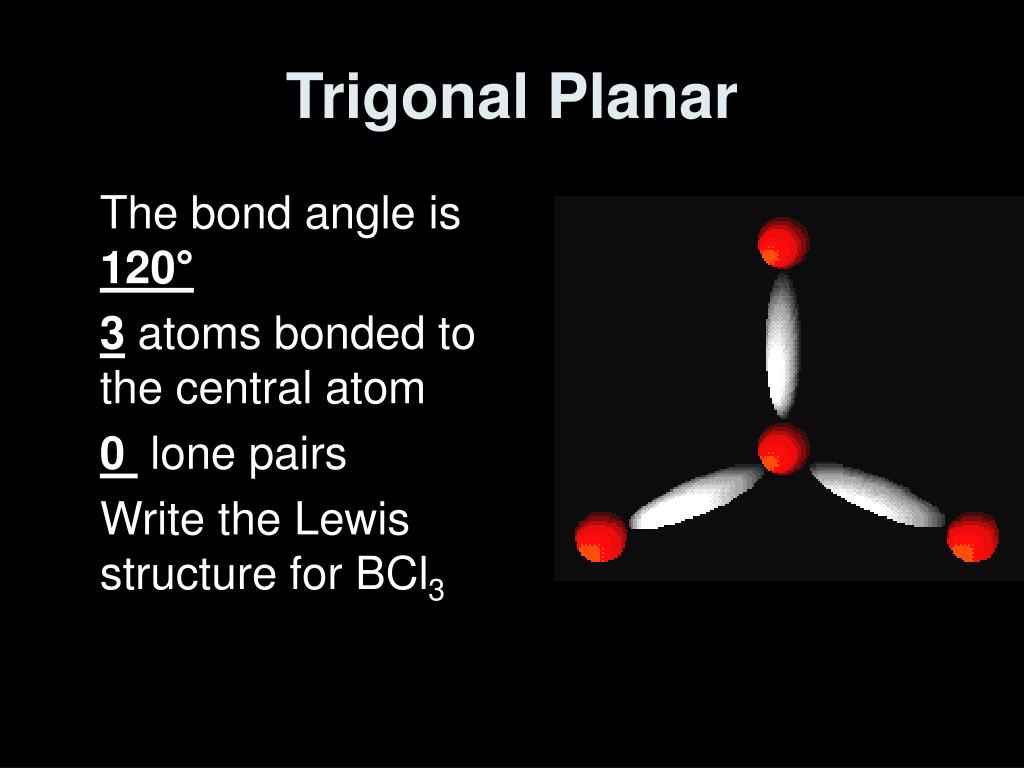
Draw Lewis Structure For Bcl3

BCl3 Lewis Structure How to Draw the Lewis Structure for BCl3 YouTube
Web A)Draw A Lewis Structure For Bcl3 (Remember That B Is A Frequent Exception To The Octet Rule).
Web The Lewis Structure Of Bcl3 Contains Three Single Bonds, With Boron In The Center, And Three Chlorines On Either Side.
Next Lets Draw The Basic Framework Of The Molecule:
Bcl3 Bcl2H Bclh2 This Problem Has Been Solved!
Related Post: