Draw The Lewis Structure For Bro3-
Draw The Lewis Structure For Bro3- - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web this problem has been solved! How many resonance structures are there in the ion? Draw the lewis structure for bro3 check all that apply. This problem has been solved! Using formal charges to determine how many bonds to make, a different perspective. How many total equivalent likely resonance structures exist for bro3 ?? Here’s the best way to solve it. 2.7m views 11 years ago. For the hbro3 structure use the periodic table to find the total number of valence electrons for the hbro3 molecule. Draw the lewis structure for bro3 check all that apply. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web a) draw all the possible lewis structures for the following ions: At first we have to calculate the valence electrons in bromate ion. There are seven valence electrons in each bromine atom and. Web this problem has been solved! Indicate the total number of valence electrons b. How many total equivalent likely resonance structures exist for bro3 ?? D) indicate the approximate bond angles for each ion e) determine the names for the molecular geometry. And this is an anion that carries one negative charge. Give the name of the ion. Indicate the total number of valence electrons b. And this is an anion that carries one negative charge. Describe the preferred lewis structure. Here’s the best way to solve it. Web a) draw all the possible lewis structures for the following ions: For the hbro3 structure use the periodic table to find the total number of valence electrons for the hbro3 molecule. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) #5 convert. Web a) draw all the possible lewis structures for the following ions: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For the hbro3 structure use the periodic table to find the total number of valence electrons for the hbro3 molecule. Adding them up, we get a total of 26 valence electrons. Web. 7 in a lewis structure for a neutral molecule, the. There are seven valence electrons in each bromine atom and six valance electrons in each oxygen atom. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms #4 minimize formal charges by converting lone pairs of the atoms,. Different factors can affect the polarity of the molecule or atom. First, we determine the total number of valence electrons in the molecule. Web this problem has been solved! Describe the preferred lewis structure. 7 in a lewis structure for a neutral molecule, the. And this is an anion that carries one negative charge. How many resonance structures are there in the ion? Different factors can affect the polarity of the molecule or atom. Describe the preferred lewis structure. Web this problem has been solved! Describe the preferred lewis structure. Adding them up, we get a total of 26 valence electrons. Drawing lewis structures for bf3, pf3 and brf3; Web chemistry chemistry questions and answers draw the lewis structure for the bromate ion (bro3−)with minimized formal charges. 7 + 3 (6) + 1= 26 valence electrons. Web this problem has been solved! #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms #4 minimize formal charges by converting lone pairs of the atoms, and try to get a stable lewis structure #5 repeat step 4 again if needed,. Using formal charges to determine how. Here’s the best way to solve it. Indicate the total number of valence electrons b. Describe the preferred lewis structure. First, we determine the total number of valence electrons in the molecule. Web a video explanation of how to draw the lewis dot structure for the bromate ion, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and bond. Web a) draw all the possible lewis structures for the following ions: Using formal charges to determine how many bonds to make, a different perspective. Give the name of the ion. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms #4 minimize formal charges by converting lone pairs of the atoms, and try to get a stable lewis structure #5 repeat step 4 again if needed,. Web this problem has been solved! There are seven valence electrons in each bromine atom and six valance electrons in each oxygen atom. Different factors can affect the polarity of the molecule or atom. For the hbro3 structure use the periodic table to find the total number of valence electrons for the hbro3 molecule. 7 + 3 (6) + 1= 26 valence electrons. How many resonance structures are there in the ion? Breaking the octet rule ;
Pin on Latest

BrO3 lewis structure, molecular geometry, bond angle, polarity, electrons
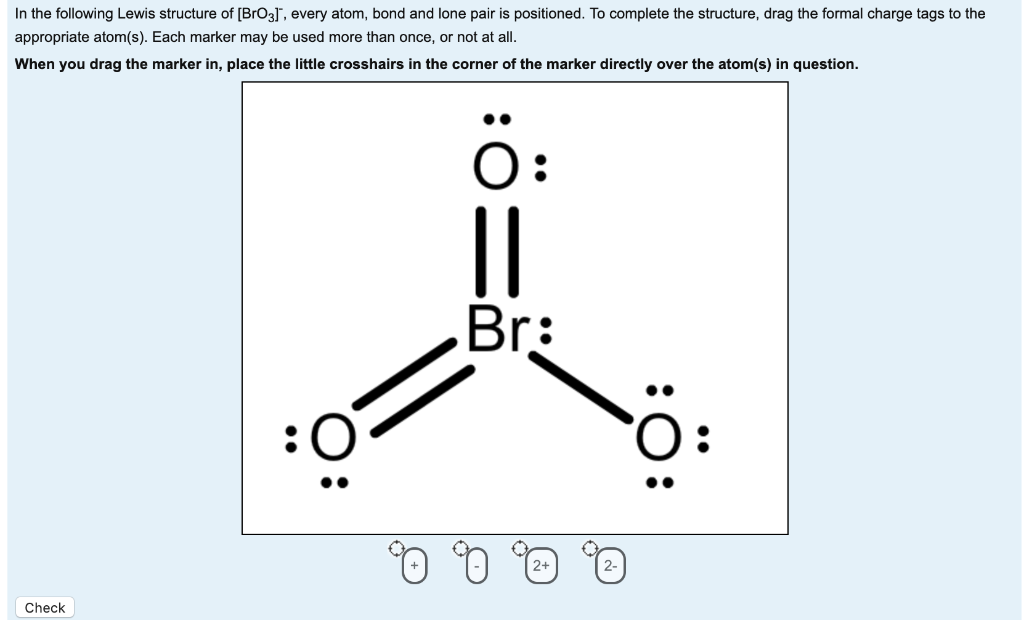
Bro3 Lewis Structure

BrO3 Lewis Structure (Bromate Ion) YouTube
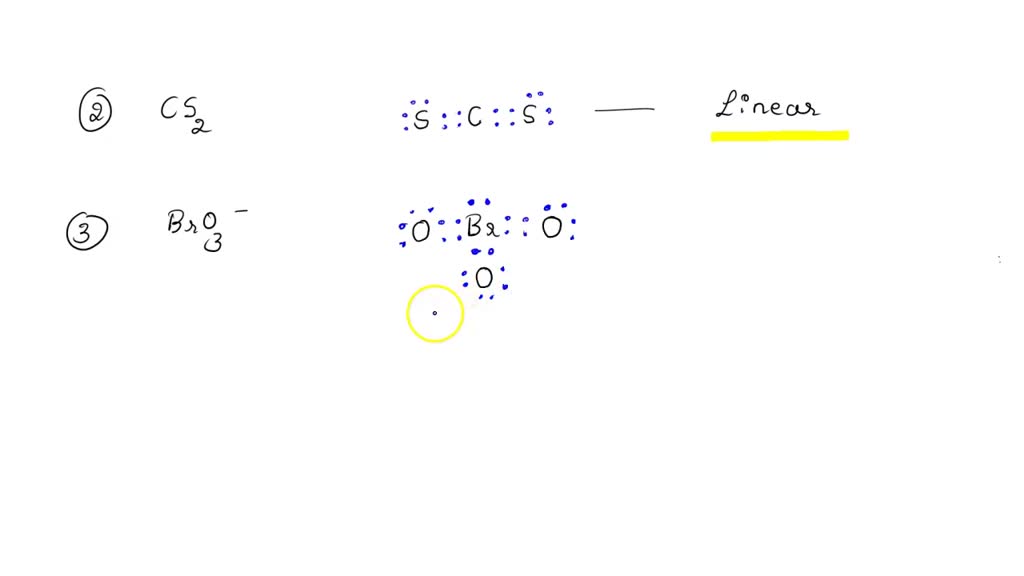
Bro3 Lewis Structure

BrO3 (Bromate ion) Molecular Geometry, Bond Angles YouTube
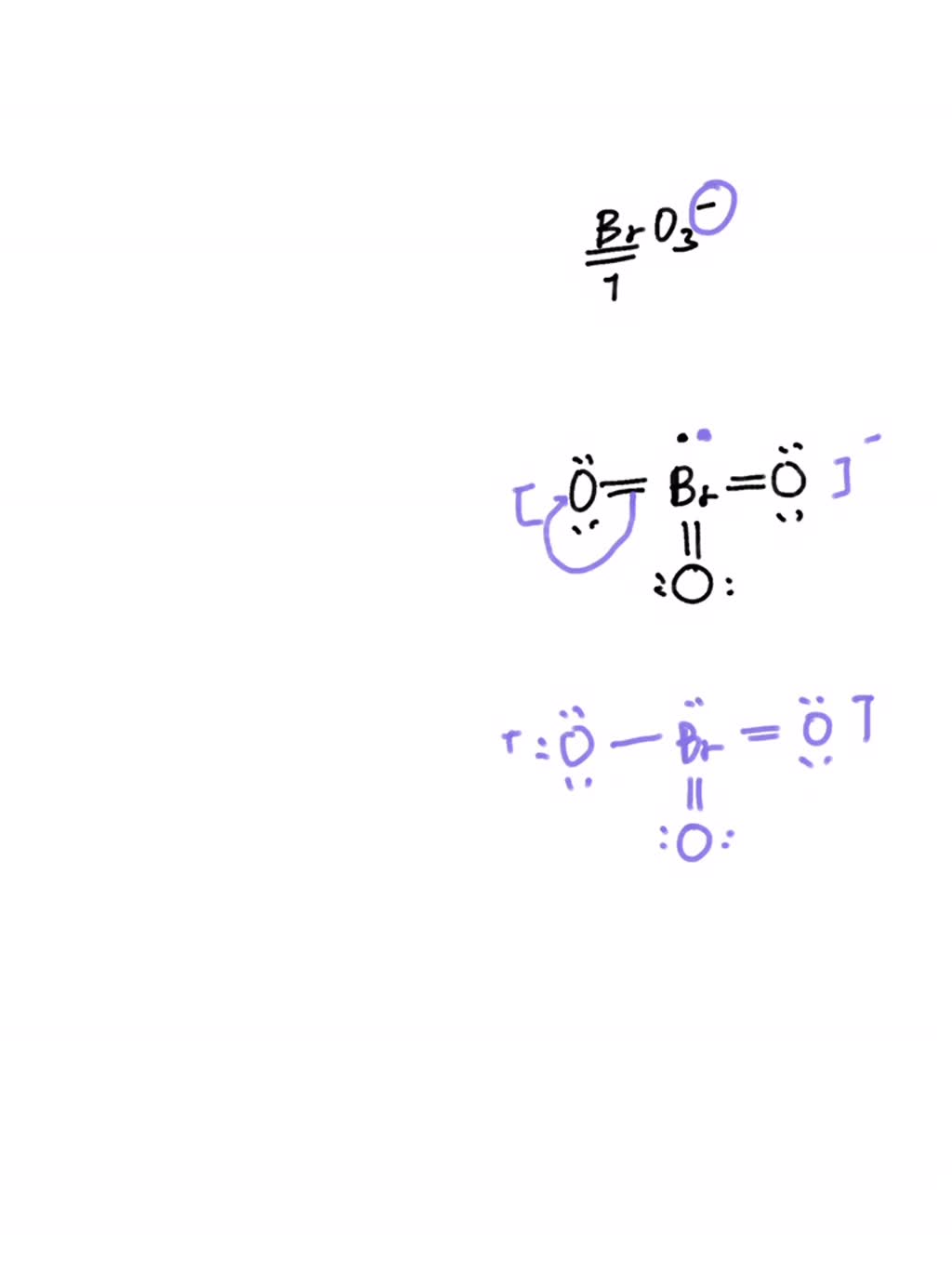
Bro3 Lewis Structure
[Solved] Draw resonance Lewis structures for bromate(V), [BrO3] −
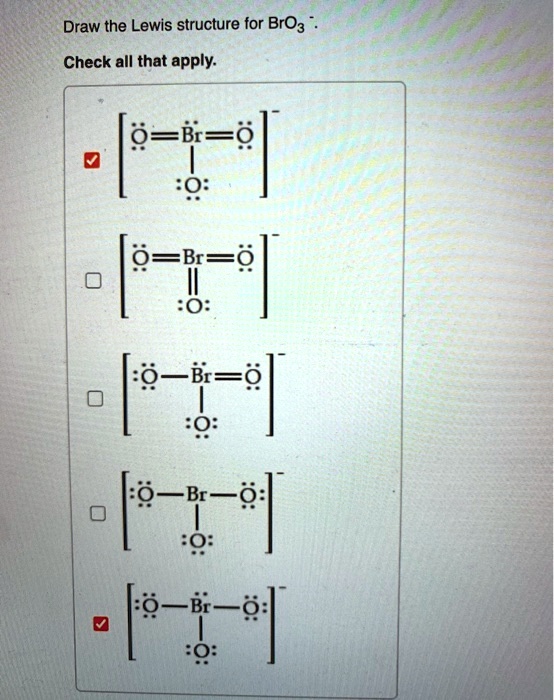
SOLVED Draw the Lewis structure for BrO3 Check all that apply 2 B=e B

BrO3 Lewis Structure How to Draw the Lewis Structure for BrO3 YouTube
So, Total Valence Electrons Will Be:
Valence Electrons Are The Outermost Electrons Of An Atom And Are Involved In Bonding.
D) Indicate The Approximate Bond Angles For Each Ion E) Determine The Names For The Molecular Geometry.
How Many Total Equivalent Likely Resonance Structures Exist For Bro3 ??
Related Post: