Draw The Lewis Structure For Of2
Draw The Lewis Structure For Of2 - Web to create the lewis structure of of2, follow these steps: Breaking the octet rule ; Using formal charges to determine how many bonds to make, a different perspective. Web answer to solved draw the lewis structure of of2, then answer Web hey guys, in this video we are going to learn about the lewis structure of of2. Here, the given molecule is of2 (oxygen difluoride). Web use these steps to correctly draw the of 2 lewis structure: Web 6 steps to draw the lewis structure of of2 step #1: Understand the proper use of the octet rule to predict bonding in simple molecules. Determine the total number of valence electrons by adding up the valence electrons of all the atoms in the molecule. Drawing lewis structures for bf3, pf3 and brf3; This molecule is made up of one oxygen atom and two fluorine atoms. Draw the atoms on paper and put dots around them to represent valence electrons of the atom. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Web write lewis symbols for. Draw the correct lewis structure of of2 and then use it to answer the questions below. [ select] b) how many lone pairs of electrons are present on. For the of2 structure use the periodic table to find the total number of valence electrons for the of2. Calculate the total number of valence electrons. Be sure to have the correct. This molecule is made up of one oxygen atom and two fluorine atoms. Web the lewis structure of xef 2 shows two bonding pairs and three lone pairs of electrons around the xe atom: Breaking the octet rule ; Using formal charges to determine how many bonds to make, a different perspective. #1 first draw a rough sketch first, determine. Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Web 6 steps to draw the lewis structure of of2 step #1: Web to create the lewis structure of of2, follow these steps: Web use these steps to correctly draw the of 2 lewis structure: The sum of the valence electrons is 5. Counting valence electrons begin by counting the total valence electrons in the molecule. Web 6 steps to draw the lewis structure of of2 step #1: Drawing lewis structures for bf3, pf3 and brf3; It is a chemical formula for oxygen difluoride. Here, the given molecule is of2 (oxygen difluoride). Thus far, we have discussed the various types of bonds that form between atoms and/or ions. Web steps of drawing of2 lewis structure step 1: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Two electrons remain, and this lone pair is placed on the xe atom: Understand the proper use of. Web the lewis structure of xef 2 shows two bonding pairs and three lone pairs of electrons around the xe atom: [ select] b) how many lone pairs of electrons are present on. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. While selecting the atom, always put the least electronegative atom. #1 first draw a rough sketch first, determine the total number of valence electrons periodic table Web the lewis structure of xef 2 shows two bonding pairs and three lone pairs of electrons around the xe atom: Web use these steps to correctly draw the of 2 lewis structure: Two electrons remain, and this lone pair is placed on the. Web 6 steps to draw the lewis structure of of2 step #1: Understand the proper use of the octet rule to predict bonding in simple molecules. Web drawing lewis structures for molecules with one central atom: This molecule is made up of one oxygen atom and two fluorine atoms. A) how many bonding pairs of electrons are in the lewis. Web how to draw lewis structure for of2? Counting valence electrons begin by counting the total valence electrons in the molecule. While selecting the atom, always put the least electronegative atom at the center. Web write lewis symbols for neutral atoms and ions. Web of2 lewis structure, molecular geometry, hybridization, polarity, and mo diagram. Using formal charges to determine how many bonds to make, a different perspective. A) how many bonding pairs of electrons are in the lewis structure? Here, the given molecule is of2 (oxygen difluoride). Web in the lewis structure of of2, both fluorine atoms share a single bond with the oxygen. Understand the proper use of the octet rule to predict bonding in simple molecules. 2f2 + 2naoh ——> of2 + 2naf + h2o. Determine the total number of valence (outer shell) electrons in the molecule or ion. Placing the least electronegative atom in the center fluorine is the most electronegative element on the. Web how to draw lewis structure for of2? Following are the steps to follow to draw the lewis. Web follow these simple steps to draw lewis dot structures: For the of2 structure use the periodic table to find the total number of valence electrons for. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. Be sure to have the correct number of electrons. Two electrons remain, and this lone pair is placed on the xe atom: #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail.
OF2 Lewis Structure How to Draw the Lewis Structure for OF2 YouTube
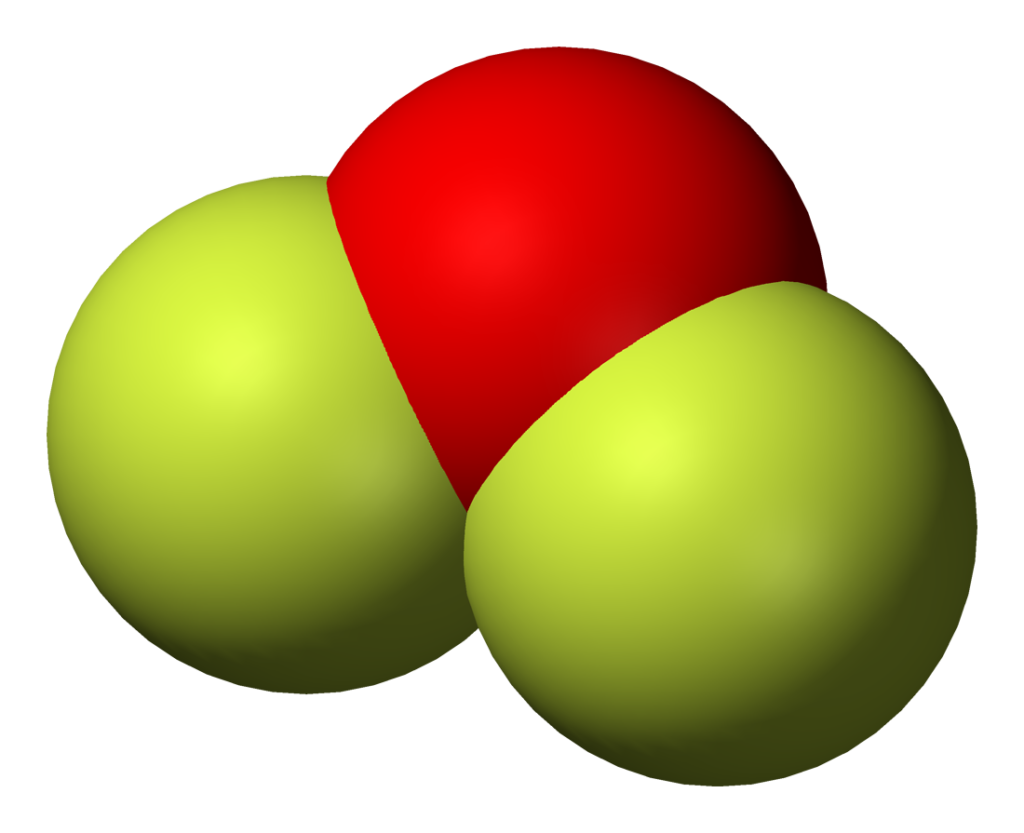
OF2 lewis structure, molecular geometry, hybridization and bond angle
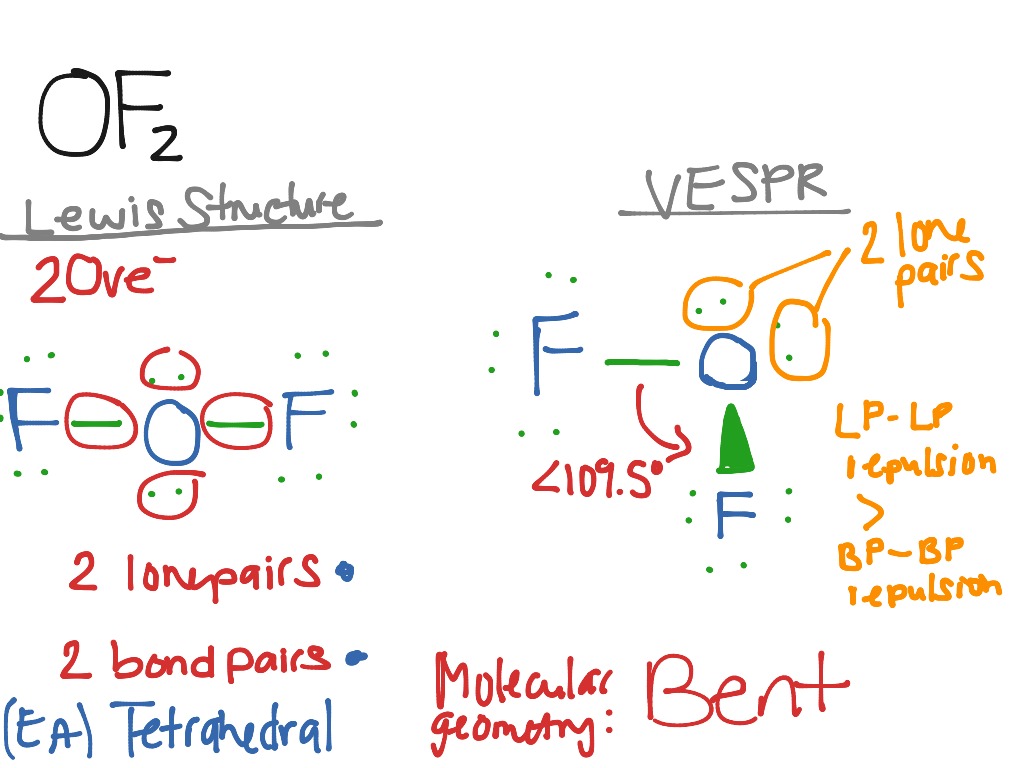
OF2 Science, Chemistry, VESPR ShowMe
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b

Drawing the Lewis Structure of OF2 (Oxygen Difluride) YouTube
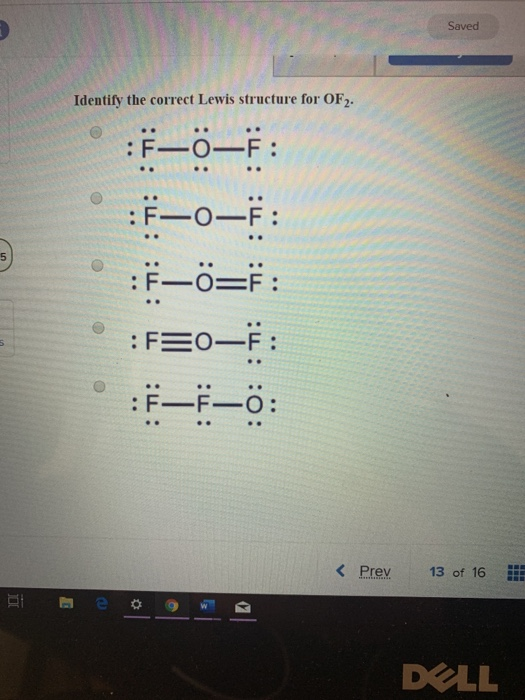
Solved Saved Identify the correct Lewis structure for OF2.
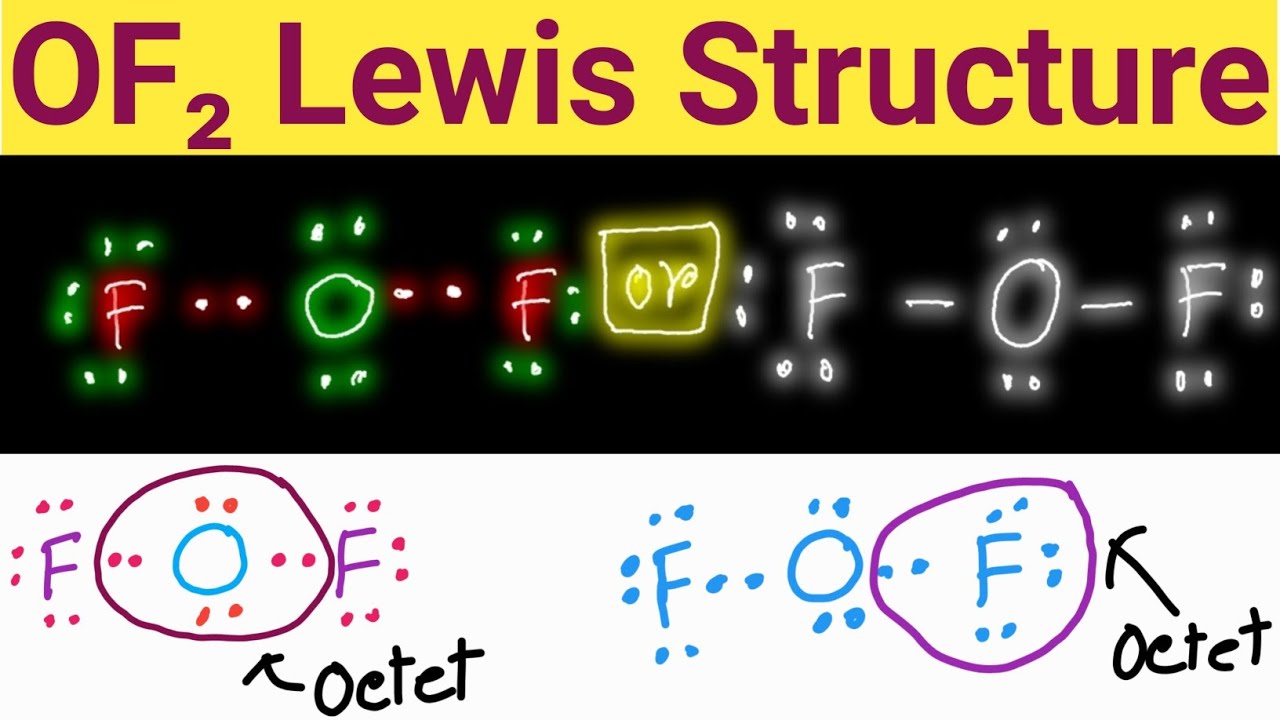
OF2 Lewis Structure Lewis Dot Structure for OF2 Oxygen Difluoride
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b
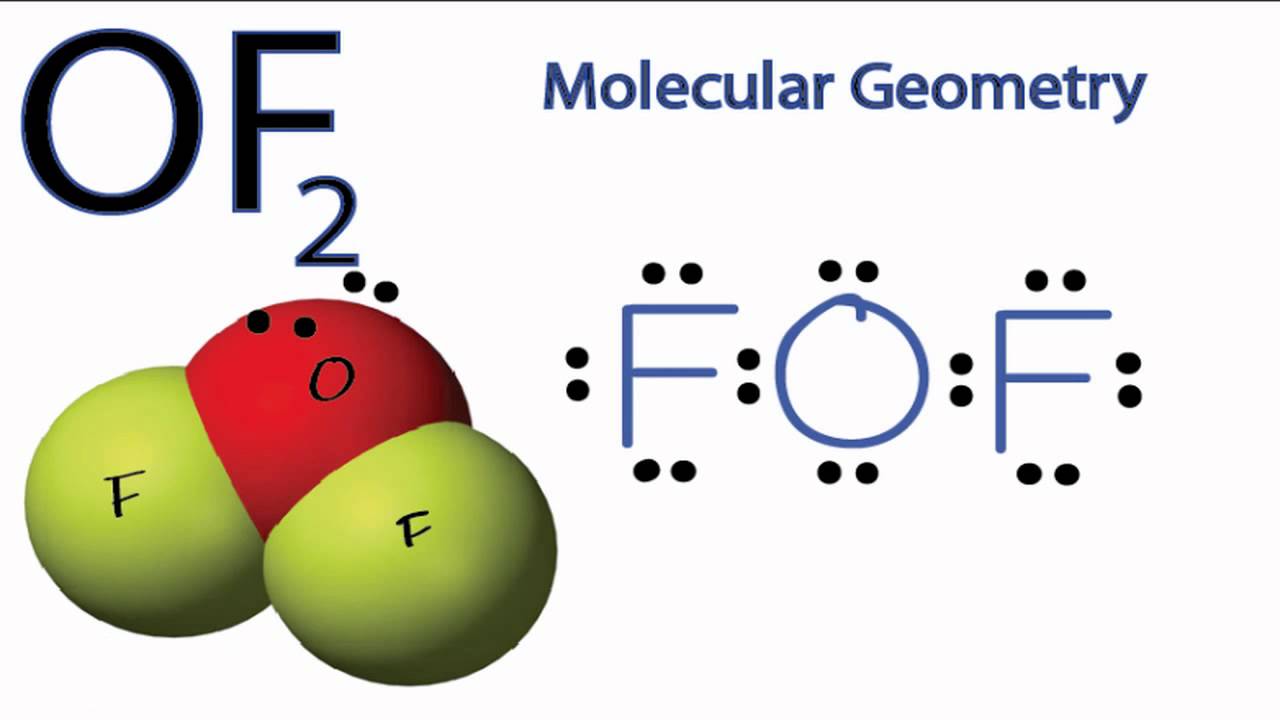
OF2 Molecular Geometry YouTube

How to Draw a Lewis Structure
Web 6 Steps To Draw The Lewis Structure Of Of2 Step #1:
Web Hey Guys, In This Video We Are Going To Learn About The Lewis Structure Of Of2.
This Molecule Is Made Up Of One Oxygen Atom And Two Fluorine Atoms.
Draw The Correct Lewis Structure Of Of2 And Then Use It To Answer The Questions Below.
Related Post: