Draw The Lewis Structure For Sicl2Br2
Draw The Lewis Structure For Sicl2Br2 - Web draw and explain the lewis structure for the mg2+ ion. Web our videos prepare you to succeed in your college classes. Draw the energy level diagram for c, h, o, n. Include all lone pairs of electrons. Web the following steps should be followed to draw the lewis structure of any molecule. And to help you understand the lewis structure. Write down the electron configuration and draw lewis dot structure for each atom. This problem has been solved! To add the element si , either double click on any atom and type the element symbol, or access a periodic table of elements from the more button. Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence electrons. To add the element si , either double click on any atom and type the element symbol, or access a periodic table of elements from the more button. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. Draw the molecule by placing atoms on the grid and connecting them. Ed lone pairs of electrons. Count the total number of valence. This problem has been solved! Draw the molecule by placing atoms on the grid and connecting them with bonds. How to draw the lewis structure for sicl2br2 watch. 4 (si) + 2(7) (cl) + 2(7) (br) = 32 step 2/5 Web drawing lewis structures for molecules with one central atom: This problem has been solved! Draw the molecule by placing atoms on the grid and connecting them with bonds. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web part a draw the lewis structure for sicl,br2. Include all lone pairs of electrons. Web in the sicl 2 br 2 lewis structure, there are four single bonds around the silicon atom, with two chlorine atoms and two bromine atoms attached to it, and on each chlorine and bromine atom, there are three lone pairs. Get the free lewis. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Ed lone pairs of electrons. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more button. In order to draw the. Web steps to draw the lewis structure of. Web the following steps should be followed to draw the lewis structure of any molecule. This widget gets the lewis structure of chemical compounds. Include all ch more button. If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! Draw the energy level diagram for c, h, o, n. Web draw the lewis structure for sicl, br2. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! Web 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more draw the molecule by placing atoms on the grid and connecting them with bonds. How to draw the lewis structure for sicl2br2 watch. Write down the electron configuration and draw lewis dot structure for. 4 (si) + 2(7) (cl) + 2(7) (br) = 32 step 2/5 Part a draw the lewis structure for sicl2br2. Determine the total number of valence electrons: Web to draw the lewis structure for sicl2br2, we need to follow a process: To add the element si, either double click on any atom and type the element symbol, or access a. Let us help you simplify your studying. While selecting the center atom, always put the least electronegative atom at the. How to draw the lewis structure for sicl2br2. Web our videos prepare you to succeed in your college classes. To add the element si, either double click on any atom and type the element symbol, or access a periodic table. 4 (si) + 2(7) (cl) + 2(7) (br) = 32 step 2/5 The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more button. Web how do you draw a lewis structure for sicl_2br_2? How to draw the lewis structure for sicl2br2. While selecting the center atom, always put the least electronegative atom at the. Web cl br the lewis structure for sicl2br2 (silicon tetrachloride dibromide) can be represented as follows: Determine the total number of valence electrons: Web 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 share 226 views 1 year ago lewis structure sicl2br2 is a chemical formula for dibromo dichloro silane. Calculate the total number of valence electrons. Web drawing lewis structures for molecules with one central atom: To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more draw the molecule by placing atoms on the grid and connecting them with bonds. Web our videos prepare you to succeed in your college classes. This problem has been solved! To add the element si , either double click on any atom and type the element symbol, or access a periodic table of elements from the more button.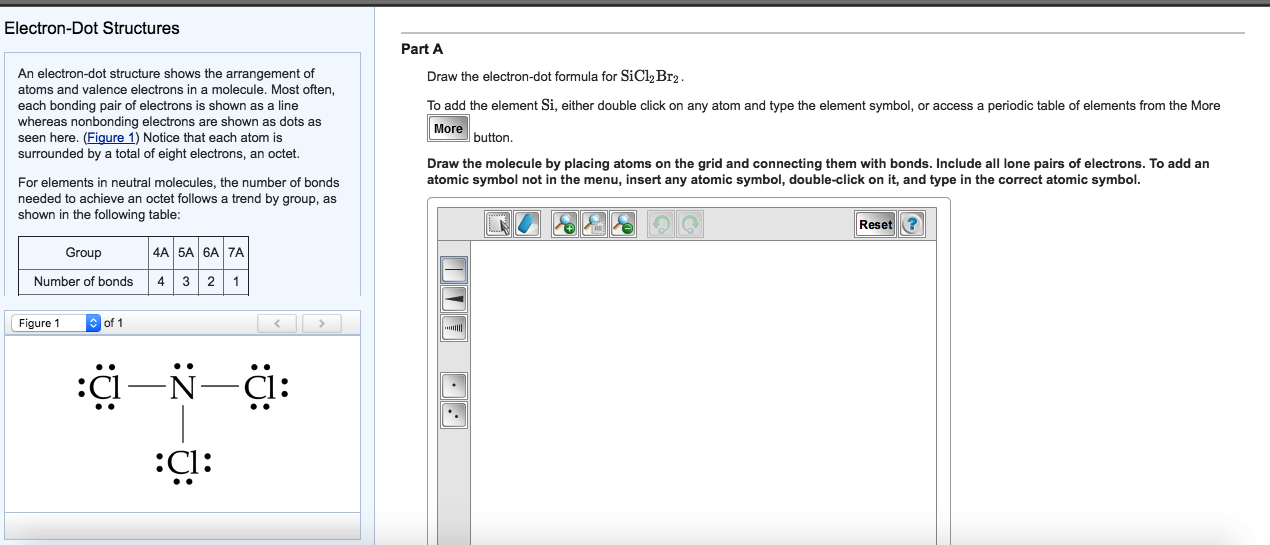
lewis structure for sicl2br2
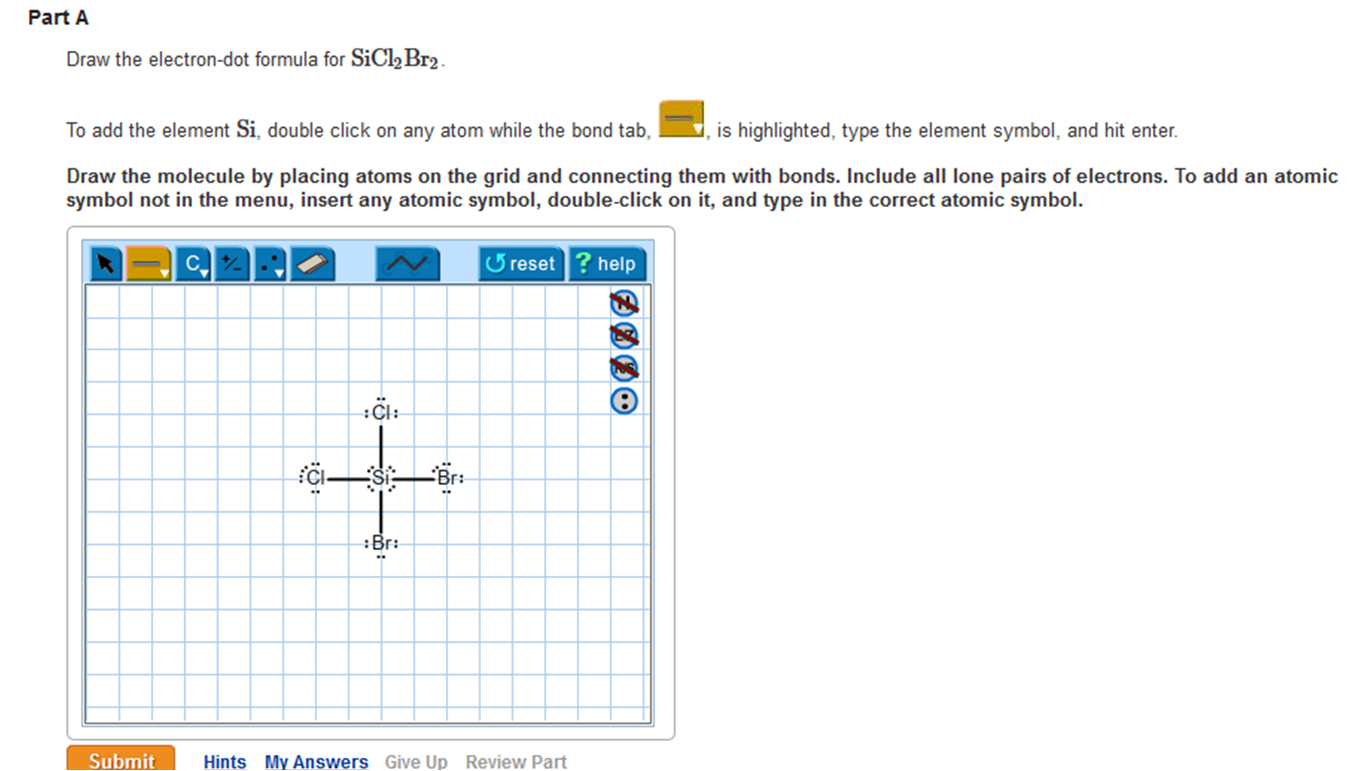
Solved Draw the electrondot formula for SiCl2Br2. This is
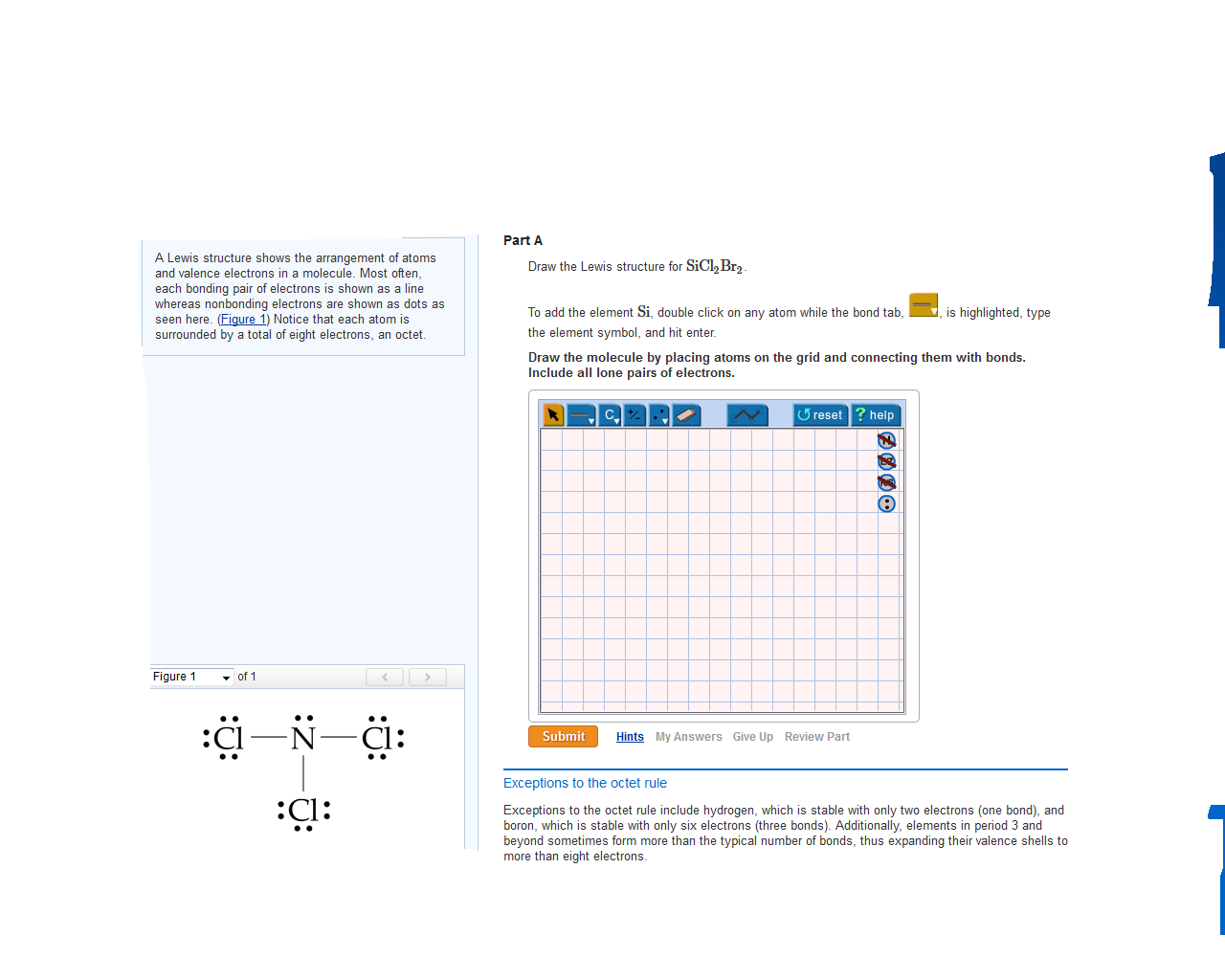
Sicl2br2 Lewis Structure How To Draw The Lewis Structure
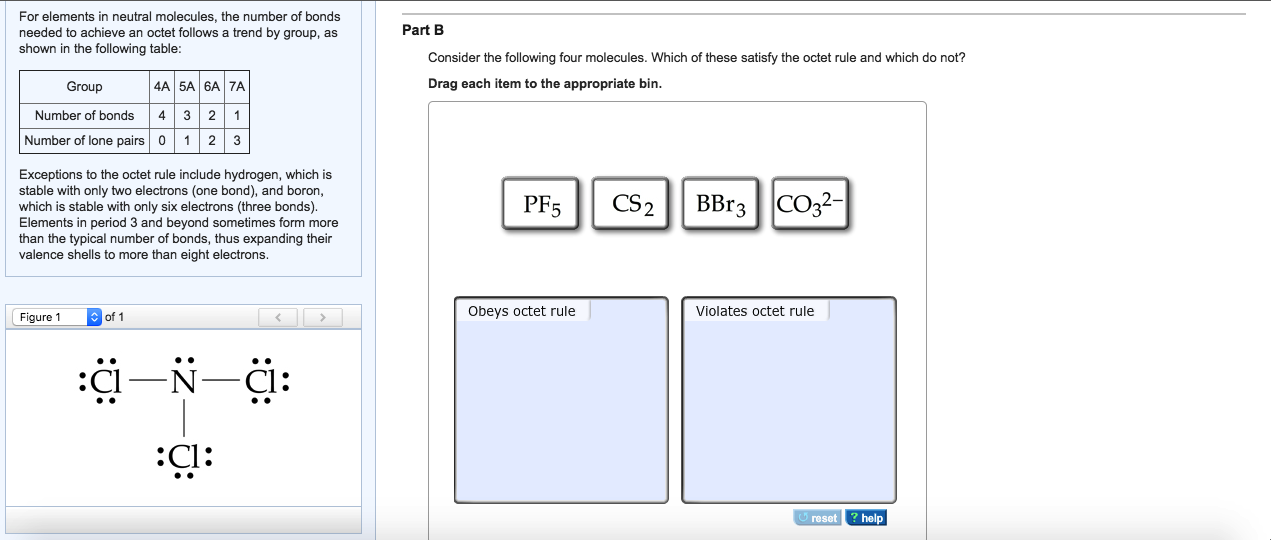
Sicl2br2 Lewis Structure How To Draw The Lewis Structure

Draw the Lewis Structure for Sicl2br2.

SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
lewis structure for sicl2br2
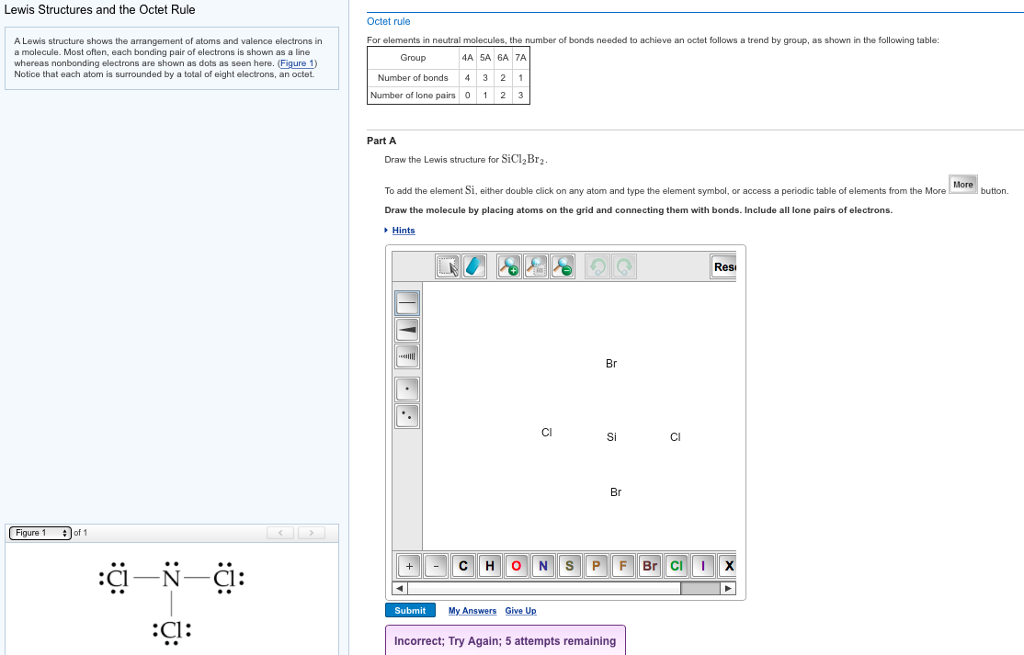
Draw The Lewis Structure For Sicl2br2
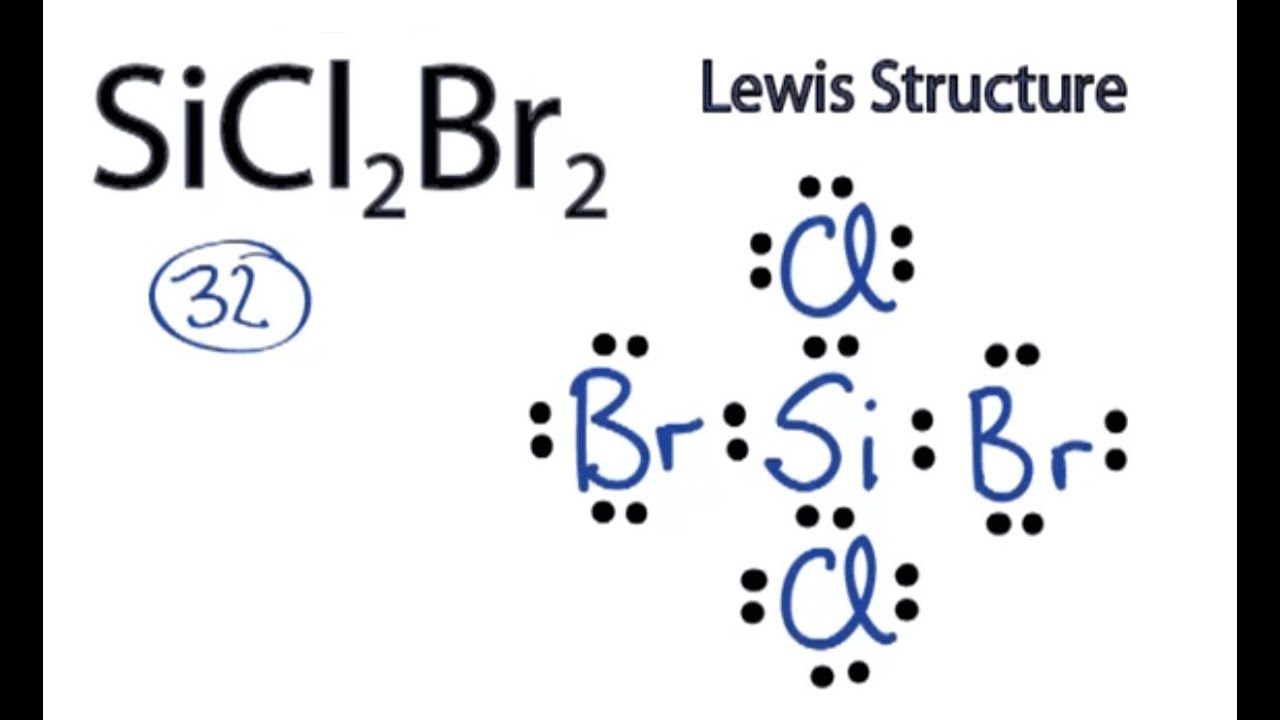
SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
lewis structure for sicl2br2
Web 6 Steps To Draw The Lewis Structure Of Sicl2Br2 Step #1:
In Sicl 2 Br 2, Silicon, Chlorine (Cl) And Bromine (Br) Have 4,7 And 7 Electrons Respectively In Their Valance Shell.
Here, The Given Molecule Is Sicl2Br2.
Web To Draw The Lewis Structure For Sicl2Br2, We Need To Follow A Process:
Related Post: