Draw The Lewis Structure For The Pcl+4 Ion
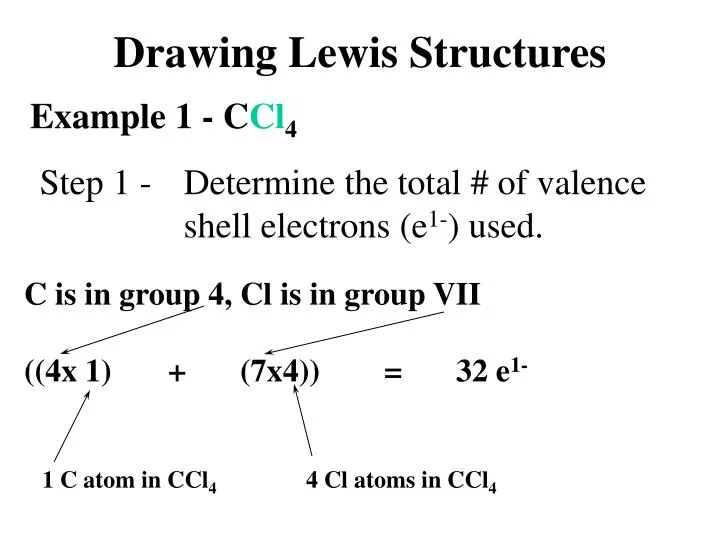
Draw The Lewis Structure For The Pcl+4 Ion - Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a. The following procedure can be used to draw lewis structure for simple molecules. For each, give (i) the molecular shape, (ii) the electron pair geometry at the central atom, and (iii) the hybridization of the central atom. For the pcl4+ structure use the periodic table to find the total number of valence electrons for the pcl4+ molecule. This problem has been solved! Phosphorous and chlorine atoms have 5. Web augchem 436 subscribers 1.8k views 3 years ago i needed to draw pcl4+ so i could show how to input it into the chemdoodle chemical drawing program in owl. Lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis structure. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Two rules in chem 101a, we will focus on drawing lewis. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis structure. This problem has been solved! Web draw the lewis structure for the pcl+4 ion. #1 draw a. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Web each chlorine (at no. First, we need to draw the lewis structures for the reactants and products. The lewis electron structure for the nh 4 + ion is as follows: #1 draw a rough skeleton structure #2 mention lone pairs on the atoms. Web lewis structure of pcl 4+. Here are the steps and final. Also, there is a positive (+1) charge on the phosphorus atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw lewis structures for covalent compounds. Web draw the lewis structure for the pcl+4 ion. Here are the steps and final. Web draw lewis structures for covalent compounds. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a. The drive for eight the octet rule refers to the tendency of. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web chemistry chemistry questions and answers draw the lewis structure for the (pcl4+)ion. Web lewis structure of pcl 4+. Also, there is a positive (+1) charge on the phosphorus atom. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3. In the lewis structure of pcl 4+, there are four single bonds around the phosphorus atom, with four chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs. Web each chlorine (at no. Show transcribed image text expert answer transcribed image text: You'll get a detailed solution from a subject matter expert that helps you. Web pcl4+ is a chemical formula for tetrachloro phosphanium ion. Draw the lewis structure for the (pcl4+)ion. The following procedure can be used to draw lewis structure for simple molecules. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web identify the number of valence electrons in each atom in the nh 4. Web draw the lewis structure for the pcl+4 ion. First, we need to draw the lewis structures for the reactants and products. Web drawing lewis structures for molecules with one central atom: Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. In the lewis structure. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. First, we need to draw the lewis structures for the reactants and products. Web drawing lewis structures. You'll get a detailed solution. For the pcl4+ structure use the periodic table to find the total number of valence electrons for the pcl4+ molecule. Cl assign a formal charge to each atom in the student's lewis structure atom formal charge left cl top cl right cl bottom cl this problem has been solved! Here are the steps and final. Web identify the number of valence electrons in each atom in the nh 4 + ion. Web pcl4+ lewis structure contains 32 total valence electrons. Web science chemistry chemistry questions and answers a student proposes the following lewis structure for the pcl4ion. Web the lewis electron structure is drawn within brackets as is customary for a molecular ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web lewis structure of pcl 4+. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a. The lewis structure for $\mathrm{pcl}_{4}^{+}$ is a phosphorus atom surrounded by four chlorine atoms, with one extra positive charge on the phosphorus atom. Ocl − is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Also, there is a positive (+1) charge on the phosphorus atom. Four pairs will be used in the chemical bonds between the p and f. The drive for eight the octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell.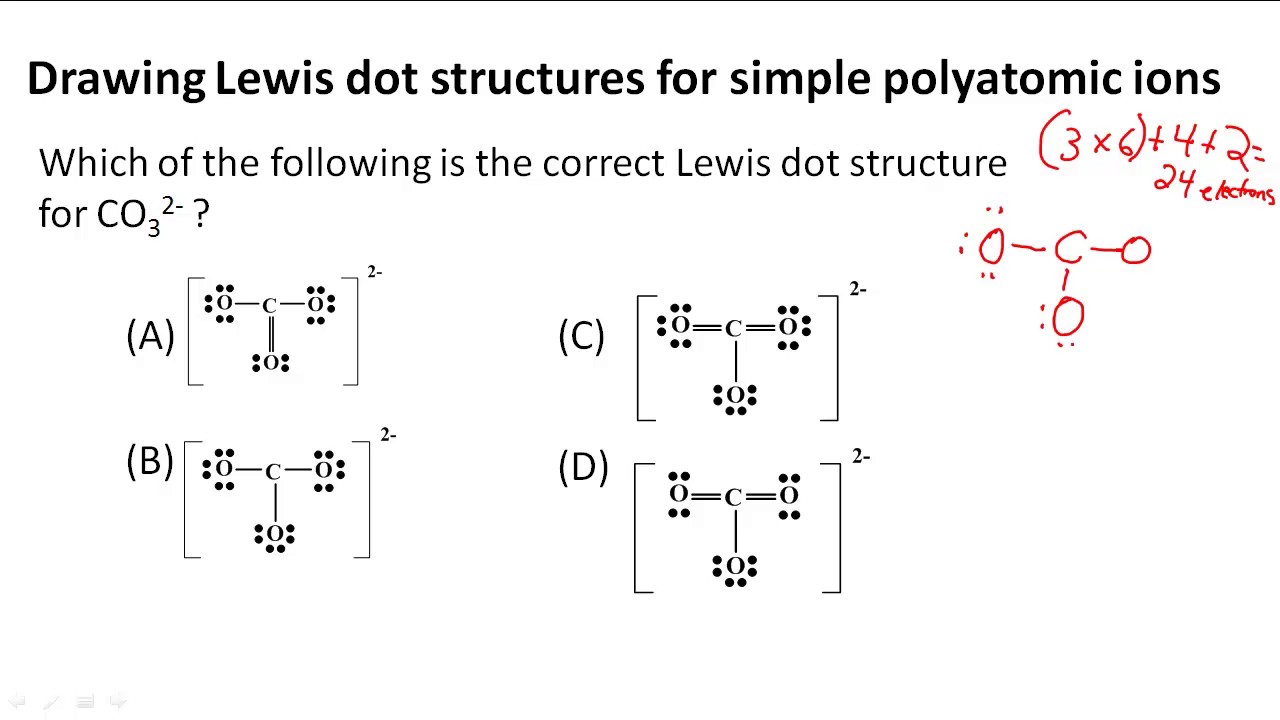
Lewis Structure Practice Worksheet
Lewis Dot Structure Definition, Examples, and Drawing
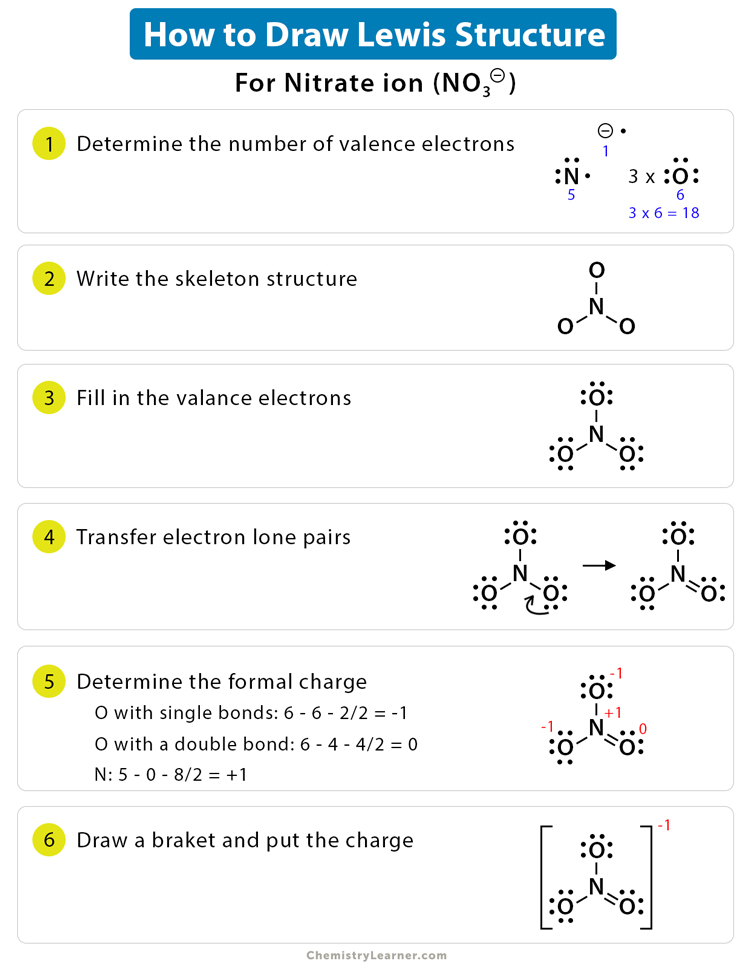
How To Draw Lewis Structures For Polyatomic Ions

How to Draw a Lewis Structure

3 Ways to Draw Lewis Dot Structures wikiHow

How To Draw Lewis Dot Structures For Compounds
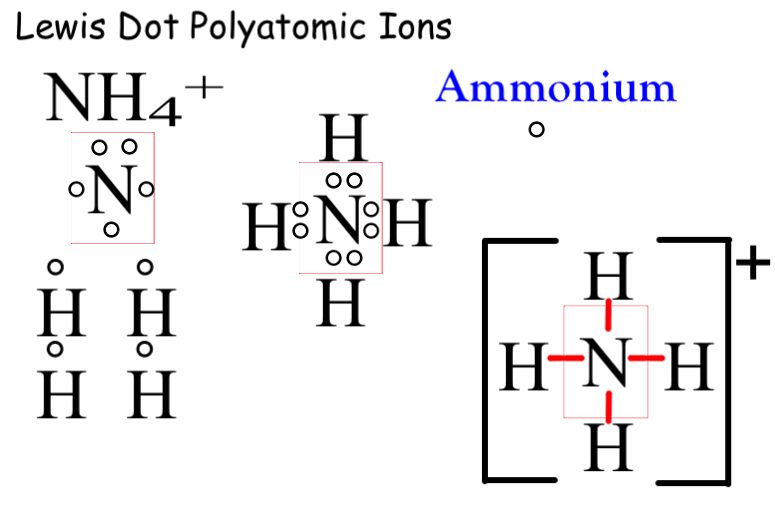
How do you draw lewis structures for polyatomic ions? Socratic

Draw the Lewis Structure for the Pcl+4 Ion

How to Draw the Lewis Structure for PCl4+ YouTube

How To Draw Lewis Structures Abilitystop
Lone Pairs, Unpaired Electrons, And Single, Double, Or Triple Bonds Are Used To Indicate Where The Valence Electrons Are Located Around Each Atom In A Lewis Structure.
Web Draw The Lewis Structure For The (Pcl_(4)^(+))Ion.
Phosphorous And Chlorine Atoms Have 5.
Web Pcl4+ Is A Chemical Formula For Tetrachloro Phosphanium Ion.
Related Post: