Draw The Lewis Structure For The Triiodide Ion
Draw The Lewis Structure For The Triiodide Ion - What is the formal charge on the central iodine atom in the structure? Web in chemistry, triiodide usually refers to the triiodide ion, i − 3.this anion, one of the polyhalogen ions, is composed of three iodine atoms. This problem has been solved! Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web the hybridization of i3 (triiodide ion) is sp3d. It will hold more than 8 electrons. It is a chemical formula for the triiodide ion. It is formed by combining aqueous solutions of iodide salts and iodine.some salts of the anion have been isolated, including thallium(i) triiodide (tl + [i 3] −) and ammonium triiodide ([nh 4] + [i 3] −).triiodide is. The ion has a linear shape, with the three iodine atoms arranged in a straight line. We then distribute these electrons around the iodine atoms, ensuring that each iodine atom has an octet of electrons. Web drawing lewis structures for molecules with one central atom: Try to show the bond polarities using dipolar moment arrow and explain your reasoning. Draw the lewis structure for the triiodide (4) 3 ion. A few salts of the anion have been isolated, including ammonium triiodide ( [nh4]+[i3]− and thallium (i) triiodide (tl+ [i3]−)). The ion is made up of. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Draw the lewis structure for the triiodide (4) 3 ion. Web i have seen the structure of triiodide ion ( ix3x− i x 3 x −) but i cannot understand why this structure is even possible. It is a common reagent. It is a chemical formula for the triiodide ion. Web solution for draw the lewis structure for the triiodide ion. Using a polarity argument, identify which of the following molecule(s) is (are) polar. Here, i’ll tell you how. Trending now this is a popular solution! It will hold more than 8 electrons. The ion has a linear shape, with the three iodine atoms arranged in a straight line. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Draw the lewis structure for the triiodide (4) 3 ion. It is a common reagent in chemistry, often. Draw the lewis structure for the triiodide (4) 3 ion. Web the hybridization of i3 (triiodide ion) is sp3d. The ion is made up of three iodine. Try to show the bond polarities using dipolar moment arrow and explain your reasoning. Web solution for draw the lewis structure for the triiodide ion. Try to show the bond polarities using dipolar moment arrow and explain your reasoning. Here, i’ll tell you how. And one iodine atom is located as the center atom. Determine the electron pair geometry, molecular geometry, and predict the bond angles? Each iodine atom contributes 7 valence electrons, resulting in a total of 21 valence electrons for the ion. This problem has been solved! And one iodine atom is located as the center atom. Web solution for draw the lewis structure for the triiodide ion. Web the hybridization of i3 (triiodide ion) is sp3d. Web drawing lewis structures for molecules with one central atom: A few salts of the anion have been isolated, including ammonium triiodide ( [nh4]+[i3]− and thallium (i) triiodide (tl+ [i3]−)). It is a common reagent in chemistry, often used in redox reactions and as an indicator for starch in iodometry. I does not follow the octet rule. (generally, the least electronegative element should be placed in the center.) connect each. Web drawing lewis structures for molecules with one central atom: Web a video explanation of how to draw the lewis dot structure for the triiodide ion, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and bond angles. It is formed by combining aqueous solutions of iodide salts and iodine.some salts of the anion have been. It is a chemical formula for the triiodide ion. Web 70 more lewis dot structures. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Determine the electron pair geometry, molecular geometry, and predict the bond angles? The ion has a linear shape, with the three iodine atoms arranged in a straight line. Web draw the lewis dot structure of the triiodide ion , and explain what conditions /reactants are necessary for it to form this problem has been solved! Web 70 more lewis dot structures. It is a common reagent in chemistry, often used in redox reactions and as an indicator for starch in iodometry. The ion has a linear shape, with the three iodine atoms arranged in a straight line. (generally, the least electronegative element should be placed in the center.) connect each atom to the central atom with a single bond (one electron pair). It is formed by combining aqueous solutions of iodide salts and iodine.some salts of the anion have been isolated, including thallium(i) triiodide (tl + [i 3] −) and ammonium triiodide ([nh 4] + [i 3] −).triiodide is. Iodine having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons. I does not follow the octet rule. I have seen in my textbook that. It also discusses the molecular geometry, bond angle, hybridization, and form. Each iodine atom contributes 7 valence electrons, resulting in a total of 21 valence electrons for the ion. Try to show the bond polarities using dipolar moment arrow and explain your reasoning. Web drawing lewis structures for molecules with one central atom: And one iodine atom is located as the center atom. (valence electrons are the electrons that are present in the outermost orbit of any atom.). Web a video explanation of how to draw the lewis dot structure for the triiodide ion, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and bond angles.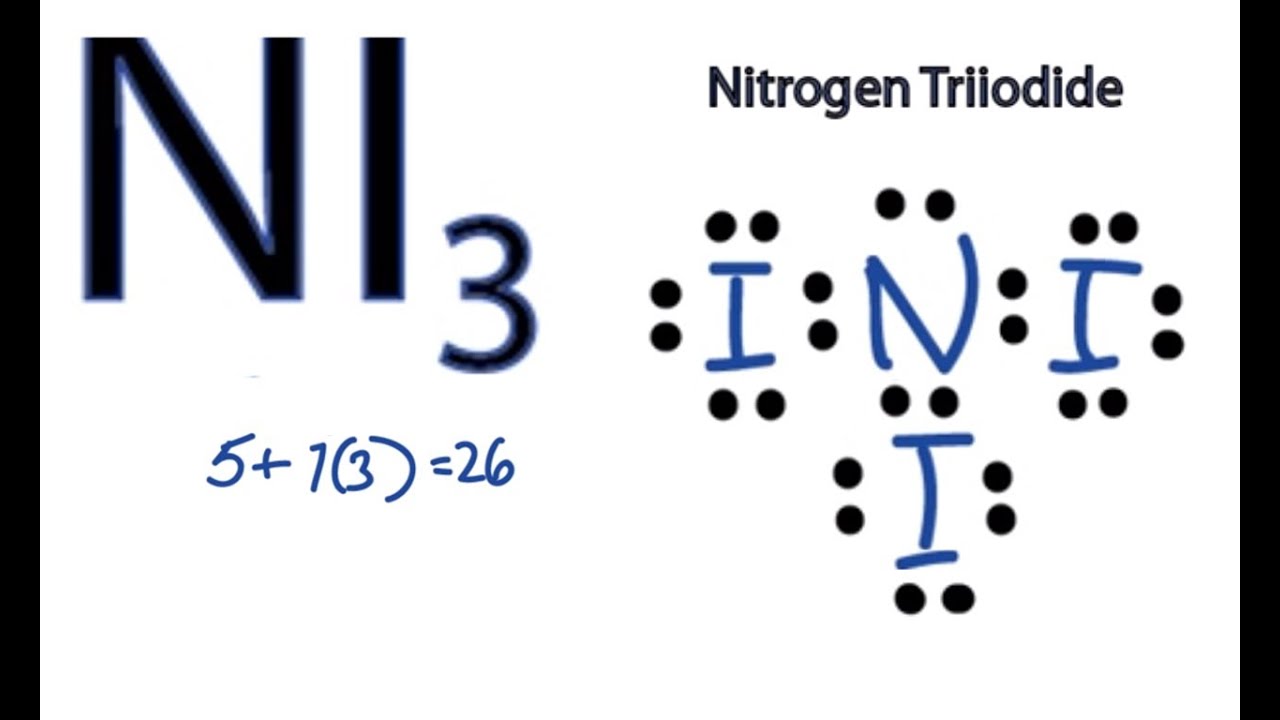
NI3 Lewis Structure How to Draw the Dot Structure for NI3 (Nitrogen

Lewis Structure of I3 (triiodide ion) YouTube

How to Draw the Lewis Dot Structure for I3 Triiodide ion YouTube

I3 Lewis Structure Triiodide Ion YouTube
Media Portfolio
![[Solved] Formal charges on polyatomic ions 9to5Science](https://i.stack.imgur.com/ikEqR.jpg)
[Solved] Formal charges on polyatomic ions 9to5Science

Lewis Dot Structure of I3 (Triiodide Ion) YouTube
Nitrogen Triiodide Lewis Structure

AP08.10 Lewis Electron dot structure of the triiodide ion YouTube

Molecular Geometry of I3 Joe Roberts
Figure Out How Many Electrons The Molecule Must Have, Based On The Number Of Valence Electrons In Each Atom.
The Ion Is Made Up Of Three Iodine.
Trending Now This Is A Popular Solution!
Here, I’ll Tell You How.
Related Post:
