Draw The Lewis Structure Of Ammonia Nh3
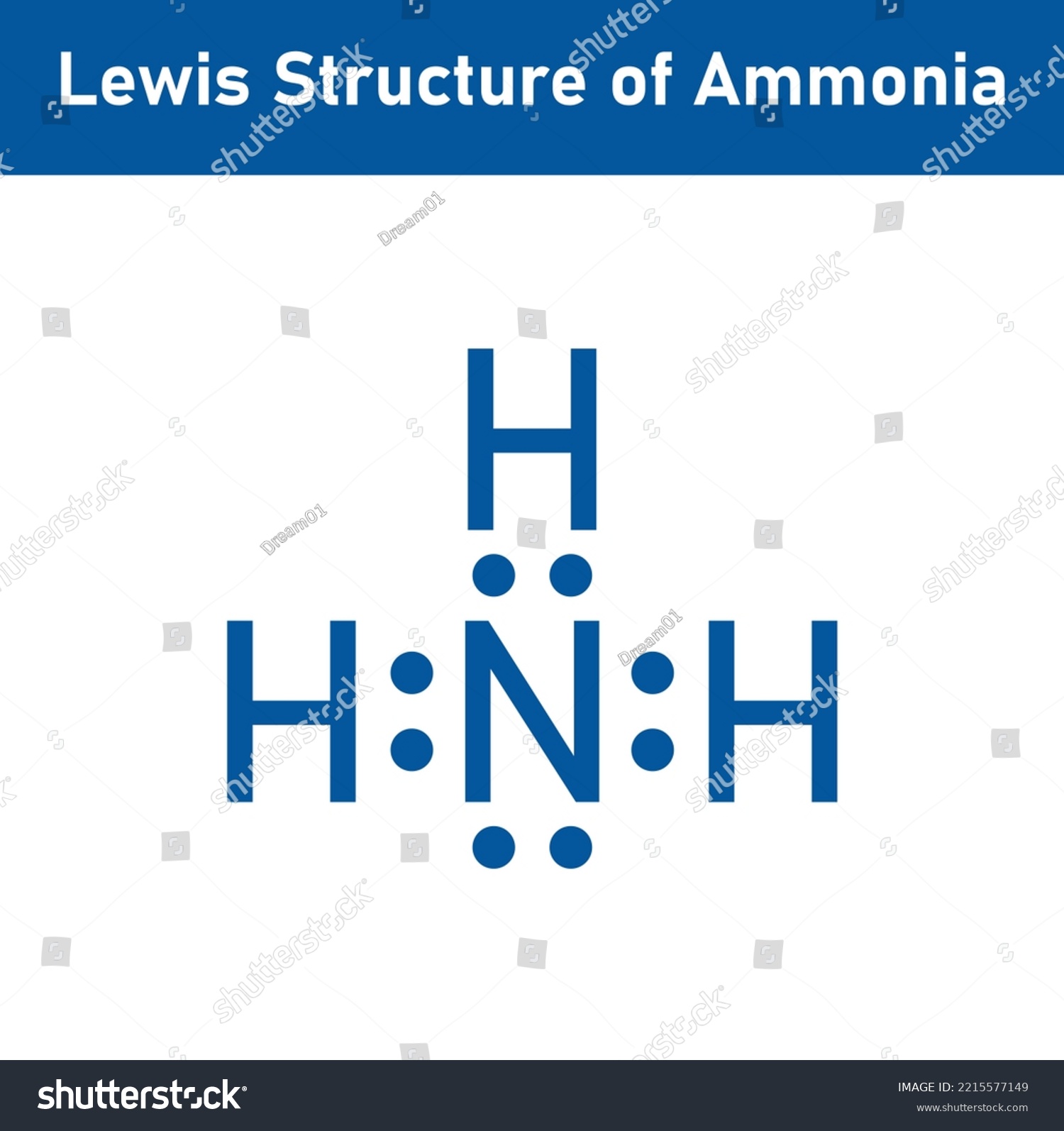
Draw The Lewis Structure Of Ammonia Nh3 - Web ammonia nh3 lewis dot structure shadowboy220 1.9k subscribers subscribe 16k views 11 years ago chemistry lewis dot structures a video explanation of how to draw the lewis dot structure. Count total valence electrons in nh3 first of all, determine the valence electron that is available for drawing the lewis structure of nh 3 because the lewis diagram is all about the representation of valence electrons around atoms. Web welcome to warren institute! Web we draw lewis structures to predict: Web ammonia (nh 3) lewis structure. Web learn more understanding the nh3 lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. Web steps of drawing nh3 lewis structure step 1: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw the lewis structure of ammonia (nh3). Number of electron regions around nitrogen atom in ammonia For resonance structures there must be a double or triple bond present, which is not the case. Here, the given molecule is nh3 (ammonia). Answer link have a look here. Find the total valence electrons in nh3 molecule in order to find the total valence electrons in nh3 molecule , first of all you should know the valence electrons present. Moreover, the presence of a single lone pair of electrons on the nitrogen atom is responsible for the bent geometrical structure of the nh3 molecule. Draw a lewis structure for ammonia (nh3). This problem has been solved! Web in this video, you will learn how to draw the lewis structure for chemicals based on total valence electrons, the octet rule,. Drawing the lewis structure for nh 3 ( ammmonia) Connect the atoms with a lone pair: Web steps of drawing nh3 lewis structure step 1: Place two dots in between the spaces found in the h's and the n. Web 15k views 3 years ago. The formula for ammonia is nh3. Web we draw lewis structures to predict: Draw a single bond between each hydrogen atom and the nitrogen atom. Here, the given molecule is nh3 (ammonia). Answer link have a look here. Find the total valence electrons in nh3 molecule in order to find the total valence electrons in nh3 molecule , first of all you should know the valence electrons present in nitrogen atom as. Web draw the lewis structure of ammonia (nh3). This is the reason why ammonia acts as a lewis base, as it can donate those electrons. You'll. Also place two dots above the n. In order to draw the lewis structure of nh3, first of all you have to find the total number of valence electrons present in the nh3 molecule. The formula for ammonia is nh3. Now, write down h, n, and h in a horizontal line. Answer link have a look here. Calculate the total number of valence electrons. Connect the atoms with a lone pair: Also place two dots above the n. Web to draw the nh3 lewis structure, follow these steps: Web 6 steps to draw the lewis structure of nh3 step #1: Web 6 steps to draw the lewis structure of nh3 step #1: Web science chemistry chemistry questions and answers draw a lewis structure for ammonia (nh3). Number of electron regions in ammonia. Count total valence electrons in nh3 first of all, determine the valence electron that is available for drawing the lewis structure of nh 3 because the lewis diagram. Web we draw lewis structures to predict: Draw a single bond between each hydrogen atom and the nitrogen atom. Number of electron regions in ammonia. There is really only one way to draw the lewis structure for ammonia (nh3). Drawing the lewis structure for nh 3 ( ammmonia) It is important to accurately distribute the valence electrons, identify the central atom, calculate formal charges, and determine bond pairs and lone pairs. This problem has been solved! Count total valence electrons in nh3 first of all, determine the valence electron that is available for drawing the lewis structure of nh 3 because the lewis diagram is all about the. Now that we know the valence electrons for the molecule, we can predict its lewis structure. + this problem has been solved! Now, write down h, n, and h in a horizontal line. Connect the atoms with a lone pair: Calculate the total number of valence electrons. Draw a lewis structure for ammonia (nh3). Place the nitrogen atom in the center of the structure and the three hydrogen atoms around it. To decide the geometry, shape and hybridization of a molecule, drawing the correct lewis structure is very important. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The lewis structure of ammonia, n h 3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. How many bonding pairs are there around the n atom? Web in this video, you will learn how to draw the lewis structure for chemicals based on total valence electrons, the octet rule, duet rule, and also you will learn about lone pair and bonding pair. Web we draw lewis structures to predict: Web learn more understanding the nh3 lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. Web welcome to warren institute! In order to draw the lewis structure of nh3, first of all you have to find the total number of valence electrons present in the nh3 molecule.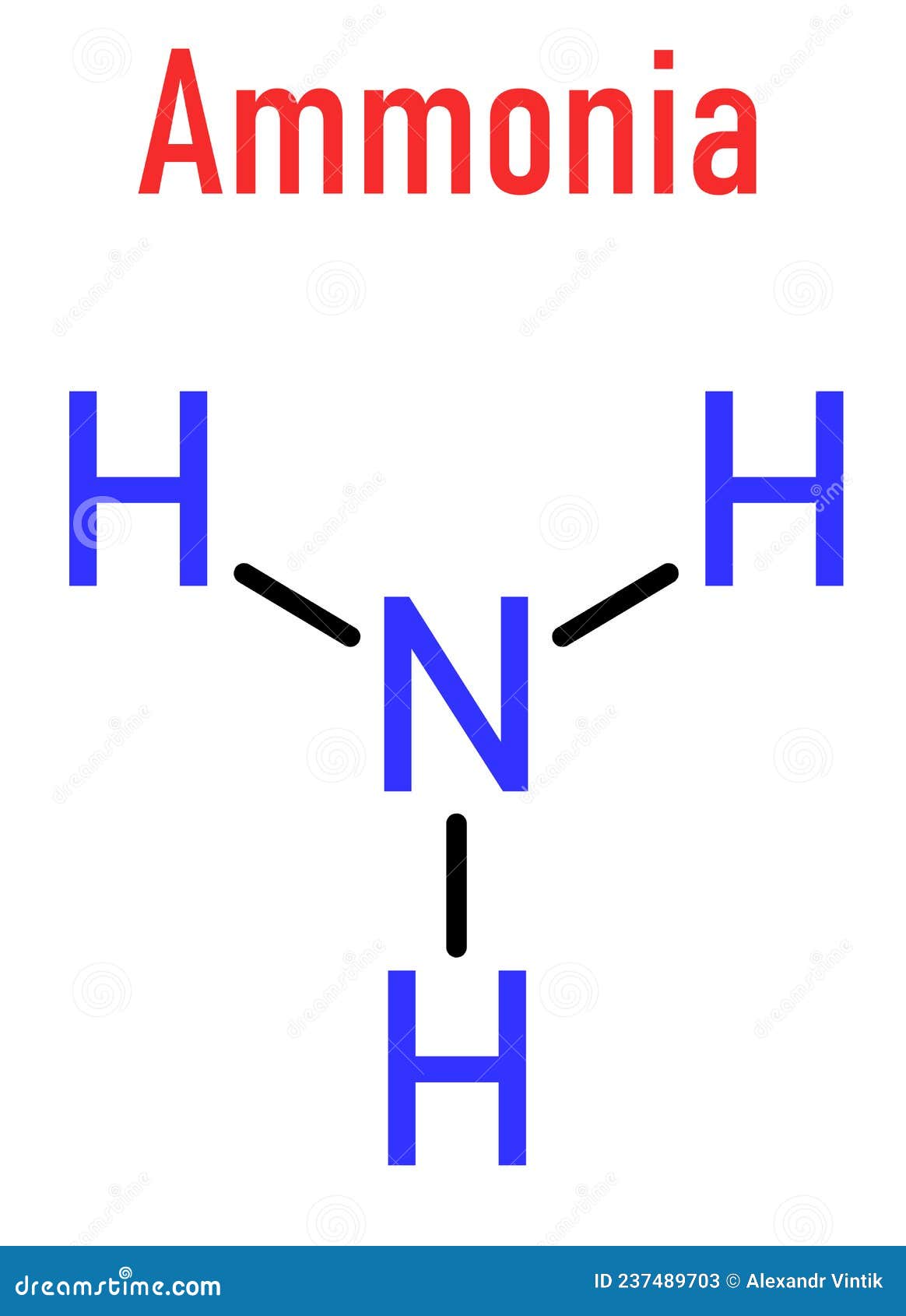
Ammonia NH3 Molecule. Skeletal Formula Stock Vector Illustration of

How to draw NH3 Lewis Structure? Science Education and Tutorials

NH3 (ammonia) Lewis dot structure YouTube
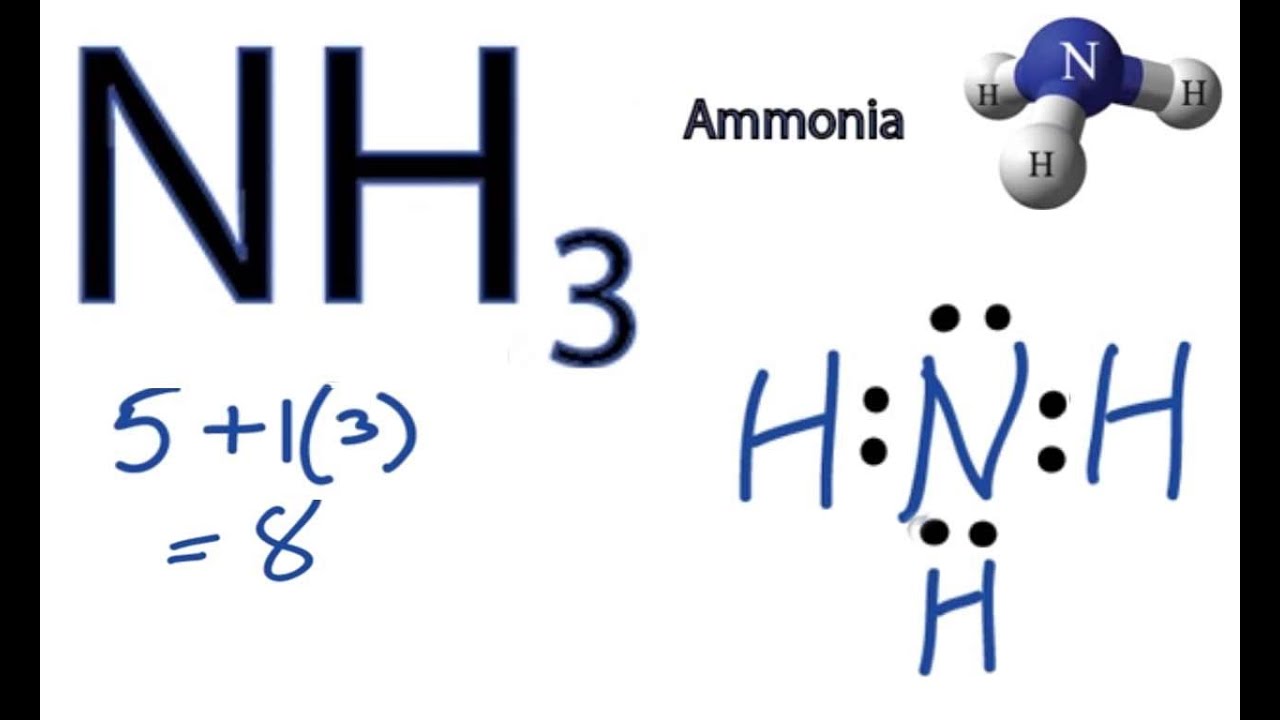
NH3 Lewis Structure How to Draw the Dot Structure for NH3 YouTube
Lewis Structure Ammonia Nh3 Scientific Vector Stock Vector (Royalty
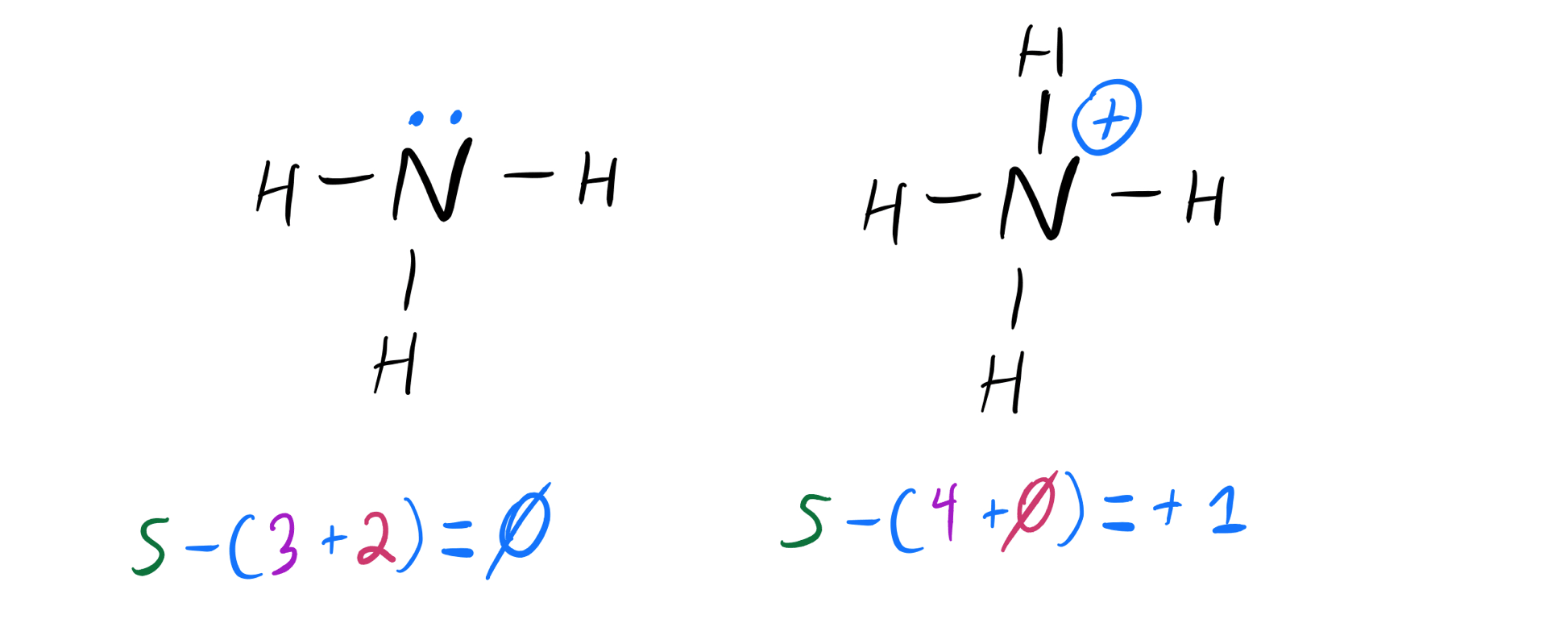
Draw the Lewis Structure for the Conjugate Acid of Ammonia En

Estructura de Lewis NH3, Amoniaco » Quimica Online

Nh3 ammonia molecule Royalty Free Vector Image

Lewis Dot Diagram Of Nh3

Lewis Structure of NH3 (Ammonia) YouTube
Web Steps Involved In The Nh3 Lewis Structure:
Drawing The Lewis Structure For Nh 3 ( Ammmonia)
Web 15K Views 3 Years Ago.
The Formula For Ammonia Is Nh3.
Related Post: