Draw The Lewis Structure Of Of2
Draw The Lewis Structure Of Of2 - For the of2 structure use the periodic table to find the total number of valence electrons for the of2. Determine the number of bonds in the molecule. Subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed to. Web the lewis structure of of2 contains two single bonds, with oxygen in the center, and two fluorines on either side. Web draw the lewis structure of of2, then answer the following questions. Web how to draw lewis structure for of2 oxygen difluoridelewis structure: Some students come to chem 101a having been taught to draw lewis structures with extra double bonds. How to draw the lewis structure for of2 watch on contents steps #1 draw skeleton #2 show chemical bond. In this case, we can condense the last few steps, since not all of them apply. Web use these steps to correctly draw the of 2 lewis structure: Web drawing the lewis structure of of2 to create the lewis structure of of2, follow these steps: Fluorine group vii, which means it has a total of seven (7. Web draw the lewis structure of of2, then answer the following questions. Since fluorine is in period 2, it can fit a maximum of eight (8) electrons second energy level. Web. Fluorine group vii, which means it has a total of seven (7. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed to. Thus far, we have discussed the various. It is a chemical formula for oxygen difluoride. Web watch on steps of drawing of2 lewis structure step 1: Web write lewis symbols for neutral atoms and ions. Web hey guys, in this video we are going to learn about the lewis structure of of2. This molecule is made up of one oxygen atom and two fluorine atoms. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. Web watch on steps of drawing of2 lewis structure step 1: Find the total valence electrons in of2 molecule in order to find the total valence electrons in of2 (oxygen difluoride) molecule, first of all you should know the valence electrons present in oxygen. Fluorine group vii, which means it has a total of seven (7. Diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Two (a pair of) valence electrons that are not used to form a covalent bond Calculate the total number of valence electrons. It is a chemical formula for oxygen difluoride. In this case, we can condense the last few steps, since not all of them apply. Web drawing the lewis structure of of2 to create the lewis structure of of2, follow these steps: Web write lewis symbols for neutral atoms and ions. Diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Web of2, aka. Understand the proper use of the octet rule to predict bonding in simple molecules. (oxygen difluroide) we draw lewis structures to predict: Web write lewis symbols for neutral atoms and ions. Identify the central atom now, we have to decipher the central atom in this molecule. Find the total valence electrons in of2 molecule in order to find the total. This molecule is made up of one oxygen atom and two fluorine atoms. There are three lone pairs on each fluorine atom, and two lone pairs on the oxygen atom. 8 + (2 × 7) = 22 xef 6: Determine the number of bonds in the molecule. Identify the central atom now, we have to decipher the central atom in. Step 2 tells how many electrons are needed and step 1 is how many electrons you have. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. How to draw the lewis structure for of2 watch on contents steps #1 draw skeleton #2 show chemical bond. Web drawing the lewis structure of of2 to create. Subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed to. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Web chemistry questions and answers. Here, the given molecule. There are three lone pairs on each fluorine atom, and two lone pairs on the oxygen atom. Web lewis structure for of. Since fluorine is in period 2, it can fit a maximum of eight (8) electrons second energy level. Draw the correct lewis structure of of2 and then use it to answer the questions below. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. 8 + (2 × 7) = 22 xef 6: Web watch on steps of drawing of2 lewis structure step 1: Web how to draw lewis structure for of2 oxygen difluoridelewis structure: Two (a pair of) valence electrons that are not used to form a covalent bond 8 + (6 × 7) = 50. Web the lewis structure of of2 contains two single bonds, with oxygen in the center, and two fluorines on either side. Understand the proper use of the octet rule to predict bonding in simple molecules. A) how many bonding pairs of electrons are in the lewis structure? Web write lewis symbols for neutral atoms and ions. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. Web of2, aka oxygen difluoride, is a molecular compound.oxygen brings 6 electrons, each fluorine brings 7 electrons, that's 20 electrons total.that's enough for.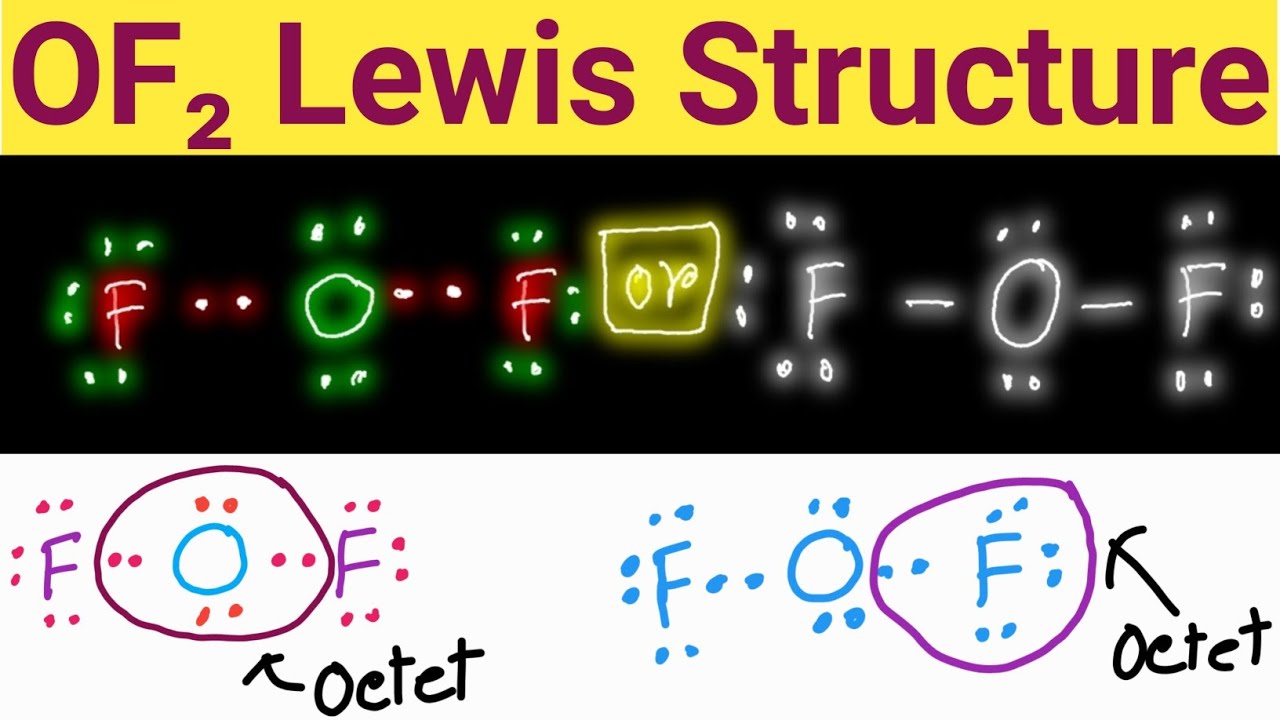
OF2 Lewis Structure Lewis Dot Structure for OF2 Oxygen Difluoride
Lewis Dot Structure for OF2 (Oxygen difluroide) YouTube

OF2 Lewis Structure How to Draw the Lewis Structure for OF2 YouTube
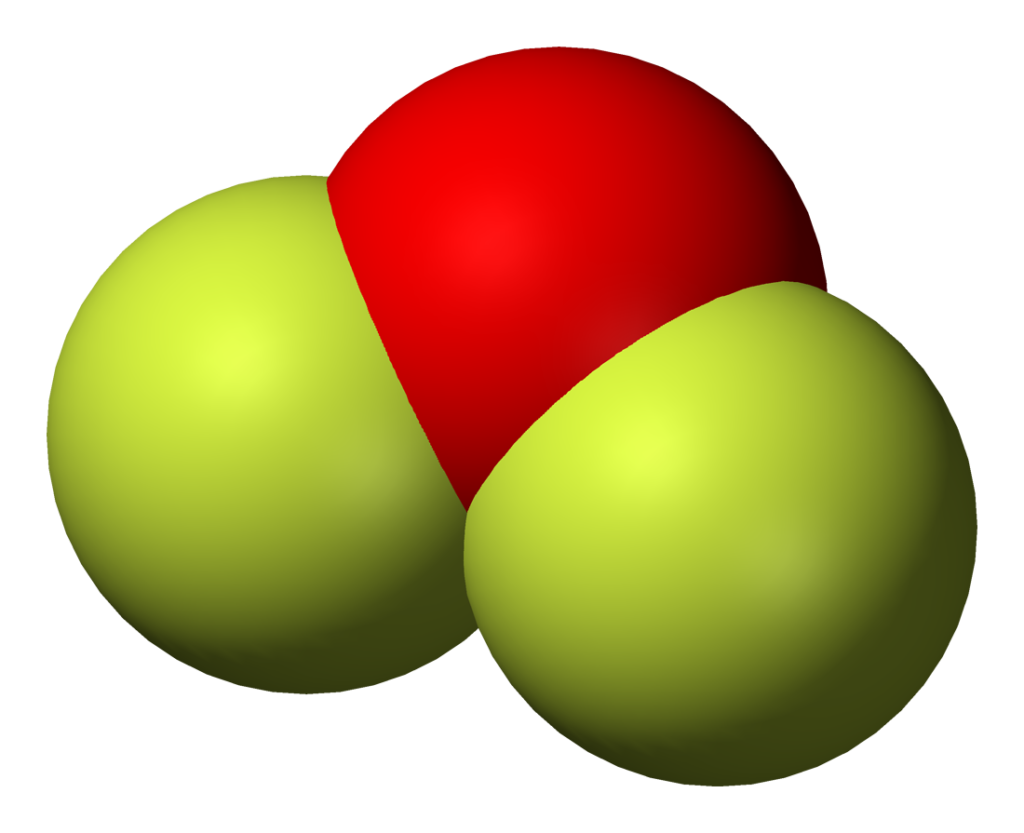
OF2 lewis structure, molecular geometry, hybridization and bond angle

OF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
[Solved] draw the lewis dot structure for the following molecule/ion
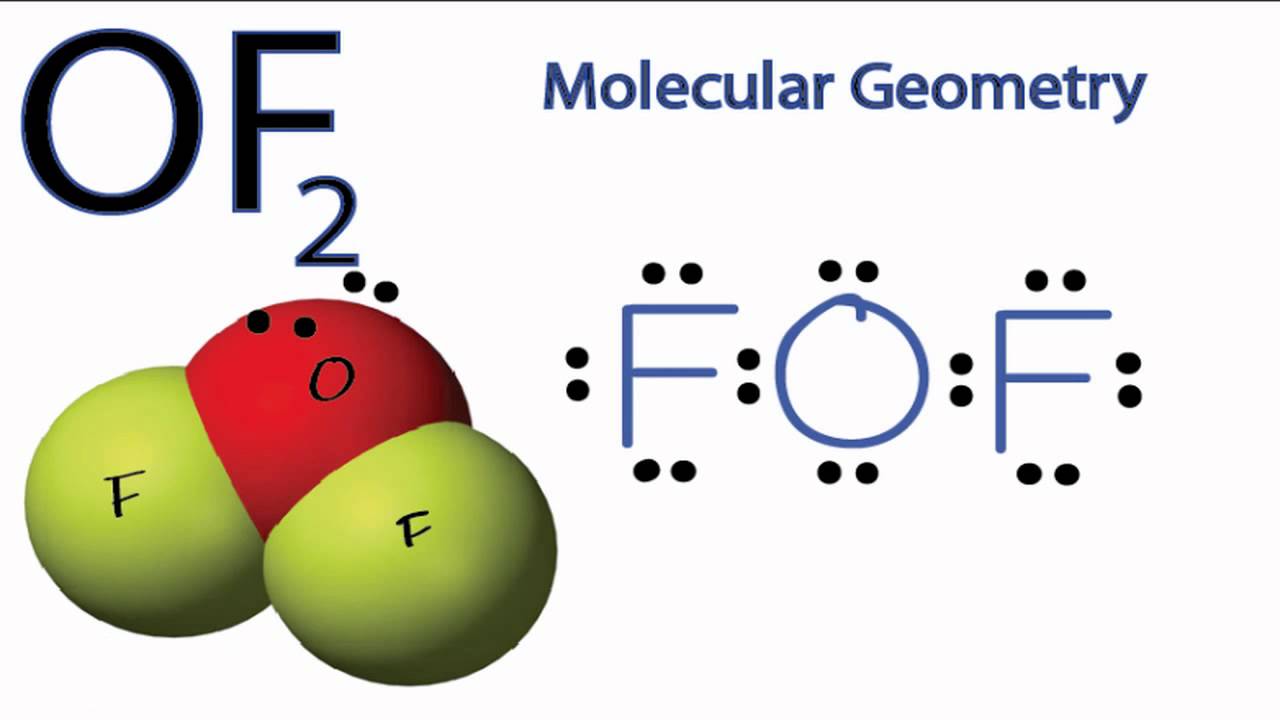
OF2 Molecular Geometry YouTube
Solved Draw the Lewis structure for OF2 and use it to
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b

Drawing the Lewis Structure of OF2 (Oxygen Difluride) YouTube
Web Drawing The Lewis Structure Of Of2 To Create The Lewis Structure Of Of2, Follow These Steps:
Sketch The Skeletal Diagram Of The Molecule In.
#1 First Draw A Rough Sketch #2 Mark Lone Pairs On The Atoms #3 Calculate And Mark Formal Charges On The Atoms, If Required Let’s Discuss Each Step In More Detail.
Web Draw The Lewis Structure Of Of2, Then Answer The Following Questions.
Related Post: