Draw The Lewis Structure Of Ph3
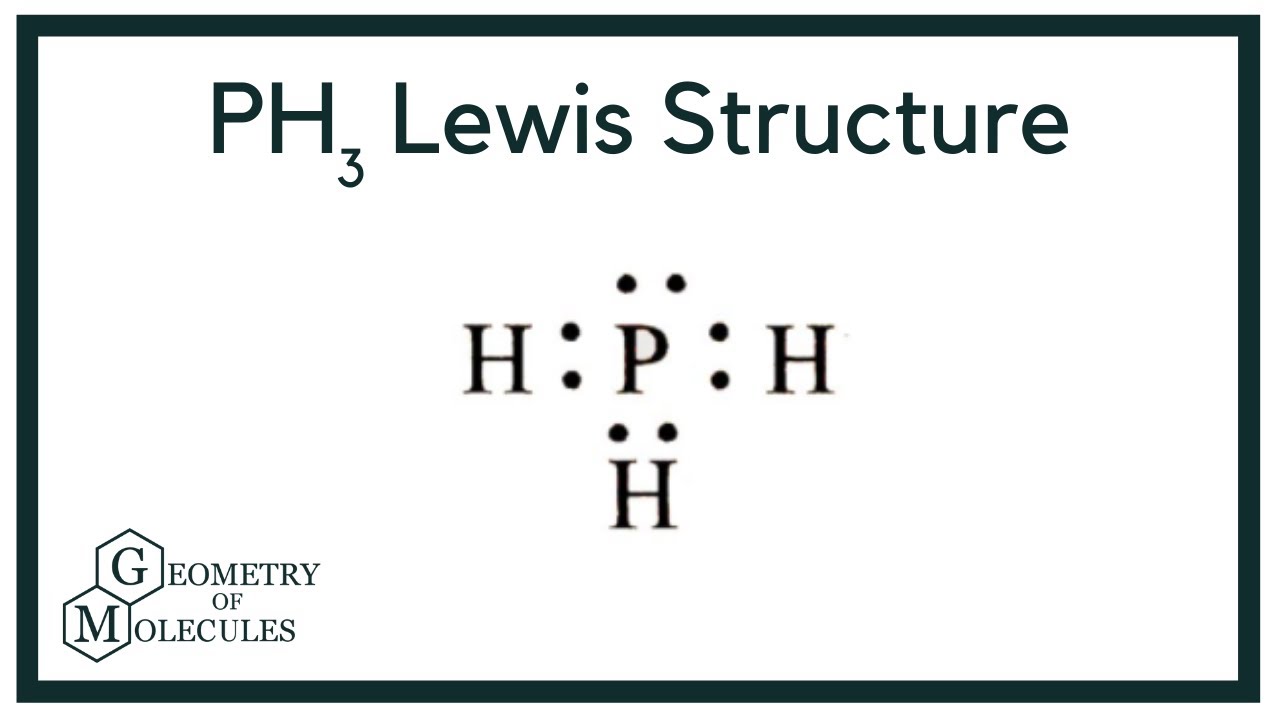
Draw The Lewis Structure Of Ph3 - In order to draw the lewis. Include lone pairs of electrons and hydrogen atoms. 8 + (2 × × 7) = 22 xef 6: Chemistry 1 answer ernest z. To add bonds connect atoms with a line draw the molecule by placing atoms on the grid and connecting them with bonds. This will be the sum of the group number a of all atoms plus the charge. Breaking the octet rule ; Web to draw the lewis structure for ph3, follow these steps: Providing for three open bonding sites. Web drawing lewis structures for molecules with one central atom: Web part a draw the lewis structure of ph3 to add lone pairs, click the button before clicking on the molecule. Breaking the octet rule ; #1 draw a rough sketch of the structure first, determine the total number of valence electrons Jan 5, 2014 here are the steps that i follow when drawing a lewis structure. Chemistry 1 answer. Web how do you draw the lewis structure for ph3? Draw a skeleton joining the atoms by single bonds. #1 draw a rough sketch of the structure first, determine the total number of valence electrons Total valence electrons pairs around phosphorous atom is four. First determine the total number of valence electrons in the molecule. Find the total valence electrons in ph3 molecule in order to find the total valence electrons in ph3 molecule, first of all you should know the valence electrons present in phosphorus atom as well as hydrogen atom. Chemistry 1 answer ernest z. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Providing for. Web contents show lewis structure of phosphene the molecular formula of phosphene is ph3 which indicates the compound has one phosphorous atom bonding with three hydrogen atoms. 8 + (2 × × 7) = 22 xef 6: Three pairs will be used in the chemical bonds between the p and h and one pair of electrons will be unbonded. Web. Web how do you draw the lewis structure for ph3? Web drawing lewis structures for molecules with one central atom: Web watch on steps of drawing ph3 lewis structure step 1: That will normally be the least electronegative atom (p). Select the center atom (h is always outside). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Include lone pairs of electrons and hydrogen atoms. If yes, then check out. Web to properly draw the ph 3 lewis structure, follow these steps: Hydrogen has an electron configuration of 1s^1. Web drawing lewis structures for molecules with one central atom: While selecting the center atom, always put the least. Web how do you draw the lewis structure for ph3? Web do you want to find out the lewis dot structure of the ph3 molecule? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The electron the steric number is pair geometry is molecular geometry is | and the this problem has been solved! Providing for three open bonding sites. This problem has been solved! #1 draw a rough sketch of the structure first, determine the total number of valence electrons While selecting the center atom, always put the least. Breaking the octet rule ; Providing for three open bonding sites. 8 + (2 × × 7) = 22 xef 6: Phosphine is a colorless and flammable gas that is commonly used in the semiconductor industry and as a reducing agent in chemical reactions. Find more chemistry widgets in wolfram|alpha. To add bonds connect atoms with a line draw the molecule by placing atoms on the grid and connecting them with bonds. In this case, we can condense the last few steps, since not all of them apply. Chemistry 1 answer ernest z. Web 6 steps to draw the lewis structure of ph3 step #1: Select the center atom (h. Decide which atom is the central atom in the structure. #1 draw a rough sketch of the structure first, determine the total number of valence electrons Using formal charges to determine how many bonds to make, a different perspective. Total valence electrons pairs around phosphorous atom is four. Therefore, it has a dot digram in the red of two electrons on top and one electron on each side. Web in the lewis structure for ph 3 there are a total of 8 valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Show transcribed image text best answer 92% (12 ratings).h:p:h. 8 + (6 × × 7) = 50; The electron the steric number is pair geometry is molecular geometry is | and the this problem has been solved! Breaking the octet rule ; This widget gets the lewis structure of chemical compounds. Find the total valence electrons in ph3 molecule in order to find the total valence electrons in ph3 molecule, first of all you should know the valence electrons present in phosphorus atom as well as hydrogen atom. Three pairs will be used in the chemical bonds between the p and h and one pair of electrons will be unbonded. Draw the lewis structure of ph3. Drawing lewis structures for bf3, pf3 and brf3;
Ph3 Lewis Structure Shape

PH3 Lewis structure YouTube

Draw The Lewis Structure Of Ph3
PH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle and

Draw The Lewis Structure Of Ph3 Fotodtp
PH3 Lewis Structure (Phosphine) YouTube

PH3 Lewis Structure How to Draw the Lewis Structure for PH3 YouTube
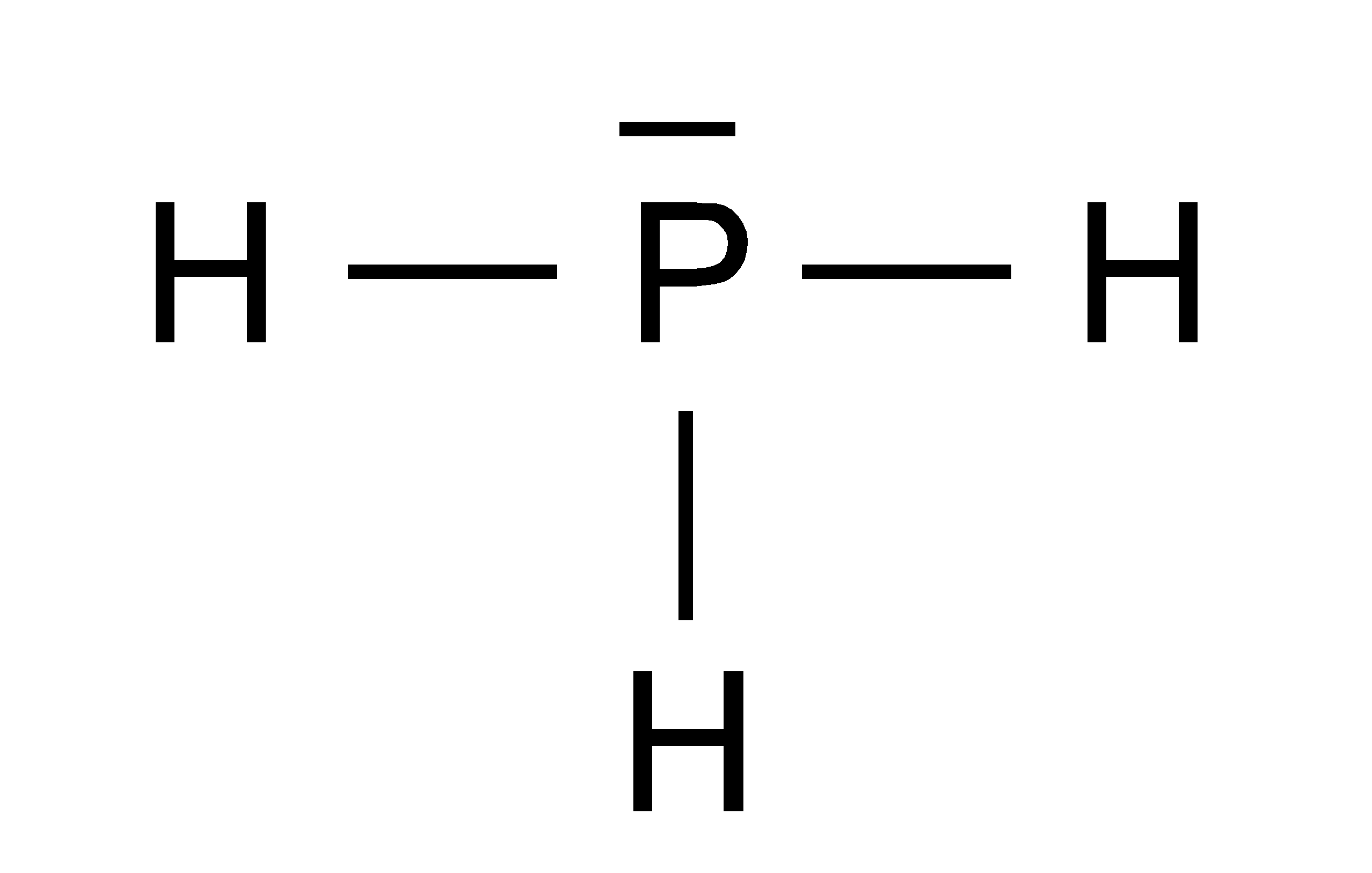
Ph3 Lewis Structure Shape

Step2 Lewis Structure of PH3 for constructing around the central

Draw the Lewis structure of PH3 YouTube
Web The Ph3 Lewis Structure Refers To The Arrangement Of Atoms And Electrons In A Molecule Of Phosphine (Ph3).
Web To Properly Draw The Ph 3 Lewis Structure, Follow These Steps:
Include Lone Pairs Of Electrons And Hydrogen Atoms.
Molecular Geometry Around Phosphorous Atom Is Tetrahedral.
Related Post: