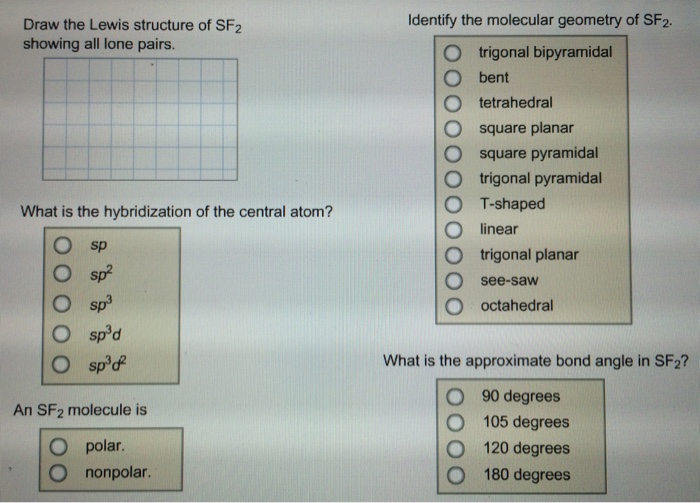
Draw The Lewis Structure Of Sf2 Showing All Lone Pairs
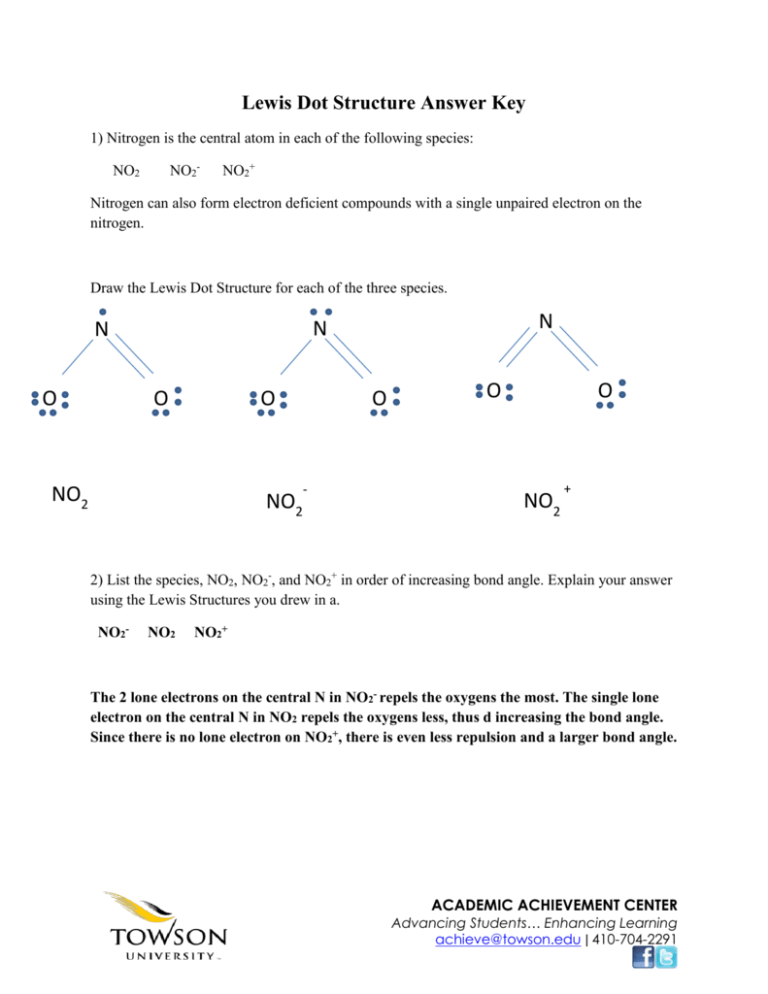
Draw The Lewis Structure Of Sf2 Showing All Lone Pairs - Draw the lewis structure of sf2, showing all lone pairs. Web drawing lewis structures for molecules with one central atom: Sulfur (s) has 6 valence electrons and each fluorine (f) has 7 valence electrons. Select draw templates more g s sp sp² sp³ d sp³ sn³d² f what is the hybridization of the central atom? Draw the lewis structure of sf2, showing all lone pairs. Electrons used in bonding = 4 In sf2, sulfur (s) is the central atom. What is the approximate bond angle in sf2? Include all the lone pairs. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web draw the lewis structure of sf, showing all lone pairs identify the molecular geometry of sf2. What is the approximate bond angle in sf2? What is the hybridization of the central atom? Draw the lewis structure of sf2. Identify the molecular geometry of sf2. Sp sp2 sp3 sp3d sp3d2 an sf2 molecule is polar, nonpolar. Web chemistry chemistry questions and answers draw the lewis structure of sf2 showing all lone pairs. Identify the molecular geometry of sf2. This problem has been solved! Number of valence electrons in two f atom = 2 (7) = 14 total number of valence electrons = 6 + 14. Sulfur (s) has 6 valence electrons and each fluorine (f) has 7 valence electrons. Draw the lewis structure of sf2. Web draw the lewis structure of sf, showing all lone pairs identify the molecular geometry of sf2. Determine the total number of valence electrons in sf2. Therefore, the total number of valence electrons in sf2 is: Select draw templates more g s sp sp² sp³ d sp³ sn³d² f what is the hybridization of the central atom? With a molar mass of 70.062 g/mol, this compound is made up of one sulfur atom and two fluoride atoms. Sulfur (s) has 6 valence electrons and each fluorine (f) has 7 valence electrons. Let's calculate the number of. Draw the lewis structure of sf2. Select draw templates more g s sp sp² sp³ d sp³ sn³d² f what is the hybridization of the central atom? Web science chemistry draw the lewis structure of sf2 showing all lone pairs. Hello students, let's begin with this question. So, the total number of valence electrons is 6 + 7 (2) =. Identify the molecular geometry of sf2. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury (||) fluoride. Identify the molecular geometry of sf2. Include all the lone pairs. So, the total number of valence electrons is 6 + 7 (2) = 20. Web place the remaining valence electron pairs on the central atom. The central atom of sulfur difluoride gets attached to the fluorine, so let's consider the solution here. Web chemistry chemistry questions and answers draw the lewis structure of sf2 showing all lone pairs. This problem has been solved! Web draw the lewis structure of sf2, showing all lone pairs. Identify the molecular geometry of sf2. Select draw templates more g s sp sp² sp³ d sp³ sn³d² f what is the hybridization of the central atom? Electrons used in bonding = 4 Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web chemistry chemistry questions and answers draw the. Place the remaining 16 electrons as lone pairs on the sulfur atom. In this question firstly we have to write down the lewis structure of sf2. Sulfur has 6 valence electrons, and each fluorine atom has 7 valence electrons. Web draw the lewis structure of sf2, showing all lone pairs. Number of valence electrons in two f atom = 2. Draw the lewis structure of sf2, showing all lone pairs. This gives the molecule a bent shape. The central atom of sulfur difluoride gets attached to the fluorine, so let's consider the solution here. With sulfur as the central atom, single bonds are formed with two fluorine atoms, with the remaining two electrons forming a lone pair on sulfur. Identify. Web place the remaining valence electron pairs on the central atom. Web chemistry questions and answers. Web draw the lewis structure of sf2, showing all lone pairs. So this sulfur this will have the 6 valence electrons and the fluorine this will have the 7 valence electrons. There are two lone pairs of electrons on the sulphur atom which makes the geometry of the molecule bent. In the hybrid orbital model, compare and contrast bonds with bonds. Place the remaining 16 electrons as lone pairs on the sulfur atom. Web drawing lewis structures for molecules with one central atom: Count the total number of valence electrons: In sf2, each fluorine atom already has an octet (8 valence electrons). Web sketch the resonance structures for the nitrite ion, no2. In this question firstly we have to write down the lewis structure of sf2. Identify the molecular geometry of sf2. Number of valence electrons in two f atom = 2 (7) = 14 total number of valence electrons = 6 + 14 = 20 electrons placing a bonding pair of electrons between the atoms to form the chemical bonds. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Sulfur has 6 valence electrons, and each fluorine atom has 7 valence electrons.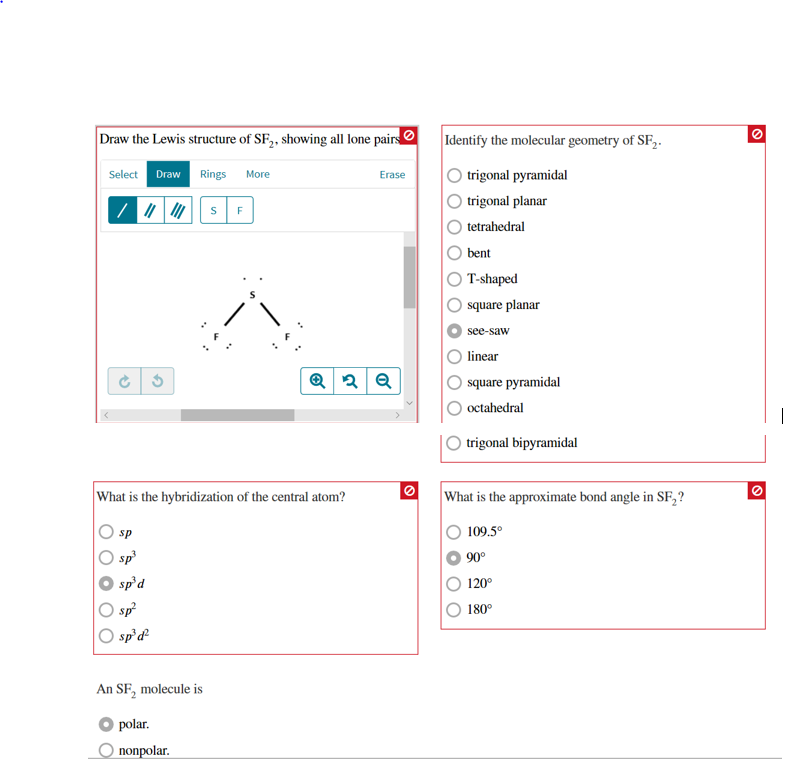
Draw the lewis structure of sf2 showing all lone pairs torbucket

Draw the Lewis structure of SF2, showing all lone pairs. Identify the
Draw the lewis structure of sf2 showing all lone pairs creativeprof

SF2 Lewis Structure How to Draw the Lewis Structure for SF2 YouTube

Draw the lewis structure of sf2 showing all lone pairs jujaeazy
SOLVED Draw the Lewis structure of SF2, showing all lone pairs
Solved Draw The Lewis Structure Of SF2 Showing All Lone P...
Draw the lewis structure of sf2 showing all lone pairs punchstart

Draw the lewis structure of sf2 showing all lone pairs punchstart
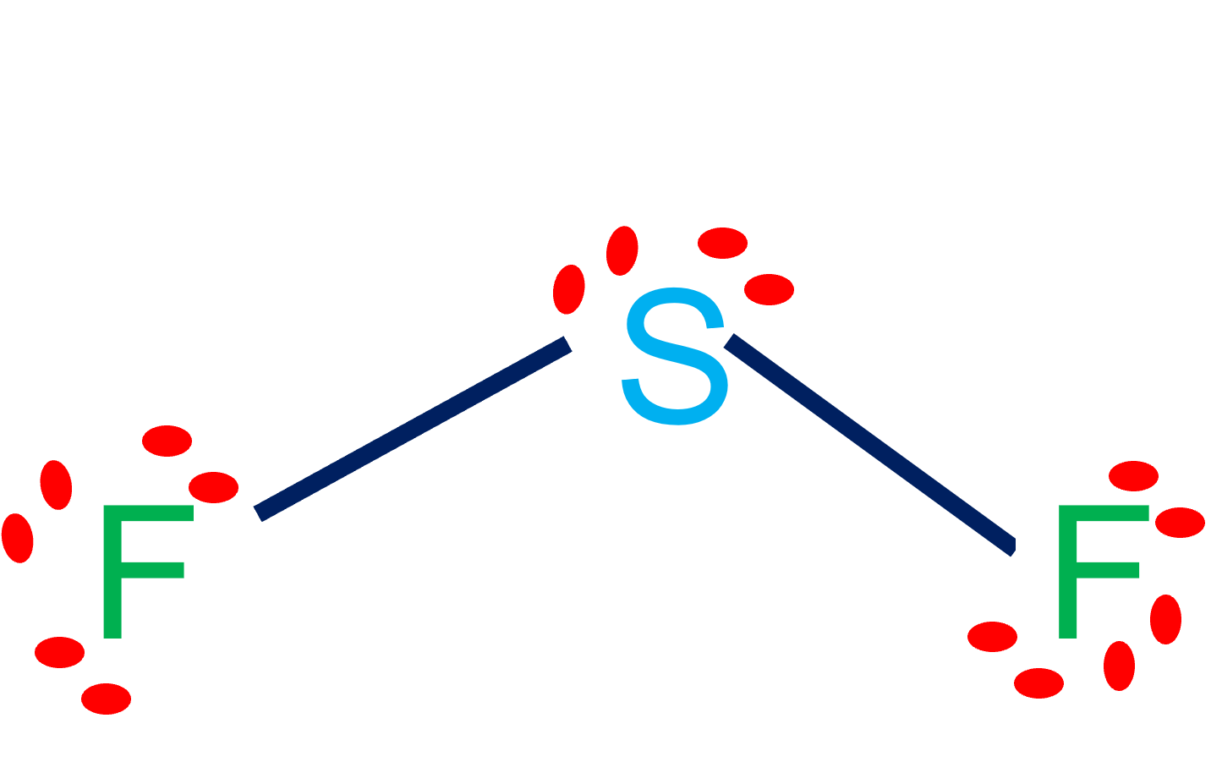
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
Draw The Lewis Structure Of Sf2.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Web Draw The Lewis Structure Of Sf2, Showing All Lone Pairs.
Identify The Molecular Geometry Of Sf2.
Related Post: