Draw The Lewis Structure Of Xef4 . Include Lone Pairs.
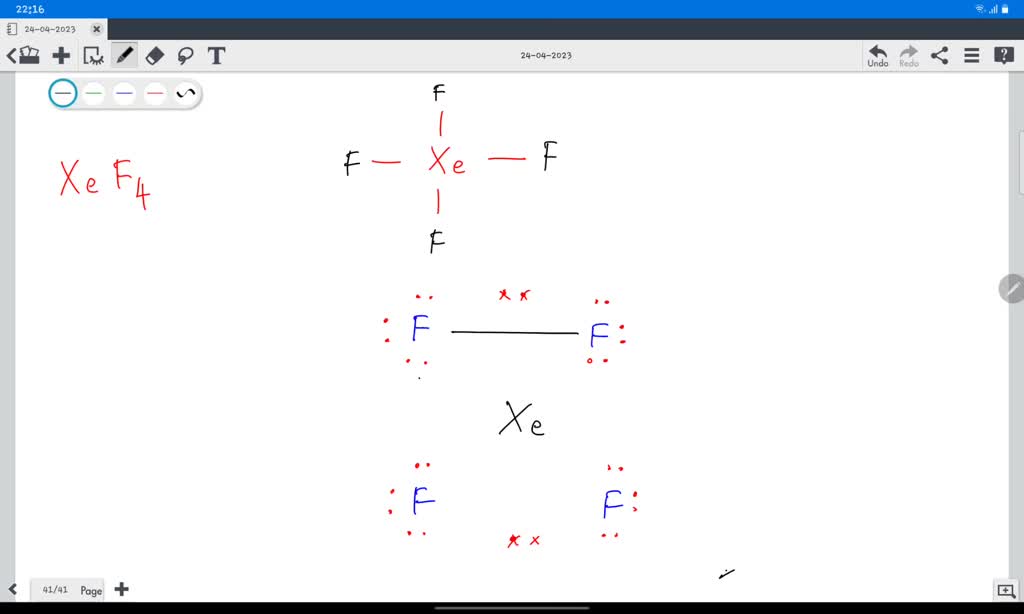
Draw The Lewis Structure Of Xef4 . Include Lone Pairs. - Identify the number of bonds and lone pairs in the structure. Web lewis structure of xef4. Web xef4 lewis structure has xenon atom (xe) at the center which is surrounded by four fluorine atoms (f). There are three lone pairs on each fluorine atom, and two lone pairs on the xenon atom. Web total valence electrons = 28 + 8 = 36 determine total valence electrons pairs total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells total electron pairs are determined by dividing the number total valence electrons by two. Web how to draw the lewis dot structure for xef4: Therefore, the vsepr theory states that there must be minimum repulsion between the electrons. 4 bonds and 6 lone pairs 4 bonds and 14 lone pairs 2 bonds and 8 lone pairs the correct answer is not shown 4 bonds and 7 lone this problem has been solved! Web may 22, 2023 by jay rana i’m super excited to teach you the lewis structure of xef4 in just 5 simple steps. Draw the molecule by placing atoms on the canvas and connecting them with bonds. Web steps here’s how you can easily draw the xef 4 lewis structure step by step: #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a closer look at each step mentioned above. There are 4 single bonds between the xenon atom (xe) and. Web chemistry chemistry questions and answers draw the lewis structure for xef4 in the window below and then answer the questions that follow. Identify the number of bonds and lone pairs in the structure. Web in xef4, xenon has two lone pairs of electrons and four bonding pairs. There are 4 single bonds between the xenon atom (xe) and each. Two fluorine atoms can form a molecule of f 2 in the same fashion. Web chemistry chemistry questions and answers draw the lewis structure for xef4 in the window below and then answer the questions that follow. Has an incomplete octet c. Web chemistry chemistry questions and answers write the lewis structure for xef4. (general chemistry) this problem has been. Therefore, the vsepr theory states that there must be minimum repulsion between the electrons. There are 2 lone pairs on the xenon atom (xe) and 3 lone pairs on all the four fluorine atoms (f). Two fluorine atoms can form a molecule of f 2 in the same fashion. The lewis structure helps us understand the bonding and electron distribution. Web the lewis dot structure shows the unpaired electrons or the lone pairs in the end. The second step is to add valence electrons to the four fluorine atoms, and the final step is to combine the step1 and step2 to get the xef4 lewis structure. Web by madhusudan dn the xeof4 lewis structure refers to the arrangement of atoms. There are three lone pairs on each fluorine atom, and two lone pairs on the xenon atom. Web draw a lewis structure for xef4 and answer the following questions based on your drawing. Web chemistry chemistry questions and answers draw the lewis structure for xef4 in the window below and then answer the questions that follow. Web may 22, 2023. Note that each atom must contribute one electron to the bond. Web chemistry chemistry questions and answers draw the lewis structure for xef4 in the window below and then answer the questions that follow. Web chemistry chemistry questions and answers xenon can be the central atom of a molecule by expanding beyond an octet of electrons. Web xef4 lewis structure. Include all lone pairs of electrons. Web steps here’s how you can easily draw the xef 4 lewis structure step by step: C what is the shape (molecular geometry) of xef4? (general chemistry) this problem has been solved! Note that each atom must contribute one electron to the bond. 4 bonds and 6 lone pairs 4 bonds and 14 lone pairs 2 bonds and 8 lone pairs the correct answer is not shown 4 bonds and 7 lone this problem has been solved! Web chemistry chemistry questions and answers write the lewis structure for xef4. You'll get a detailed solution from a subject matter expert that helps you learn. There are 2 lone pairs on the xenon atom (xe) and 3 lone pairs on all the four fluorine atoms (f). Web total valence electrons = 28 + 8 = 36 determine total valence electrons pairs total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells total electron pairs are determined by dividing the. So, if you are ready to go with these 5 simple steps, then let’s dive right into it! Web how to draw the lewis dot structure for xef4: Web the lewis dot structure shows the unpaired electrons or the lone pairs in the end. Remember that lewis structures primarily show the bonding and valence electron distribution in molecules, and Today we are going to look at the lewis structure of xef4 ( xenon tetrafluoride ) although xenon is a noble gas it reacts with four fluorine atoms to attain a stable structure. It is a type of noble gas having the chemical equation of. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Two fluorine atoms can form a molecule of f 2 in the same fashion. There are 4 single bonds between the xenon atom (xe) and each fluorine atom (f). Web chemistry chemistry questions and answers draw the lewis structure for xef4 in the window below and then answer the questions that follow. The four fluorine atoms occupy the equatorial positions , while the two lone pairs are located in the axial positions. Identify the number of bonds and lone pairs in the structure. Web xef4 lewis structure has xenon atom (xe) at the center which is surrounded by four fluorine atoms (f). The presence of lone pairs affects the molecular geometry , leading to a distorted octahedral shape. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Obeys the octet rule b.
Draw The Lewis Structure Of Xef4 Include Lone Pairs Fotodtp

XeOF4 Lewis Structure, Geometry, Hybridization, and Polarity

XeF4 Lewis Structure How to Draw the Lewis Structure for XeF4 YouTube

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram

XeOF4 Lewis Structure How to Draw the Lewis Structure for XeOF4 YouTube

XeF4 Lewis structure, Molecular geometry, Bond angle, Shape

XeF4 Lewis Structure How to Draw the Lewis Structure for XeF4 YouTube

So far, we’ve used eight of the XeF4 Lewis structure’s total 8

Step2 Lewis Structure of XeF4 for counting valence electrons around
SOLVED How many lone pairs of electrons are there in the Lewis
#1 Draw A Rough Skeleton Structure #2 Mention Lone Pairs On The Atoms #3 If Needed, Mention Formal Charges On The Atoms Now, Let’s Take A Closer Look At Each Step Mentioned Above.
Has An Expanded Octet Draw A Lewis Structure For No2.
There Are Three Lone Pairs On Each Fluorine Atom, And Two Lone Pairs On The Xenon Atom.
The First Step Is To Sketch The Lewis Structure Of The Xef4 Molecule, To Add Valence Electrons Around The Xenon Atom;
Related Post: