Draw The Orbital Diagram
Draw The Orbital Diagram - The electron configuration for phosphorus is 1s 2 2s 2 2p 6 3 s 2 3p 3 and the orbital diagram is drawn below. #3 draw orbital diagram of fluorine. Web this wave function also helps us in drawing boundary surface diagrams. You will also get the hd images of the periodic table (for free). Web here’s how you can draw the orbital diagram of fluorine step by step. Web orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. Web valence bond theory vbt. This makes it a challenge to draw, but i will show you the. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. Discover the three rules for creating electron orbital charts, and study examples of filling electron orbital in a diagram. Individual atomic orbitals (ao) are arranged on the far left and far right of the diagram. Once the atomic number has been identified, write the. Web this video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. It explains how to write the orbital diagram notation (with arrows) of an element. An orbital. Valence bond theory (vbt) in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. This makes it a challenge to draw, but i will show you the. Be 2 s, f 2 , cn − o) is cn − diamagnetic or. Web when drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. #3 draw orbital diagram of fluorine. Web write the electron configuration for phosphorus and draw the orbital diagram. Carbon (atomic number 6) has six electrons. Web text 5 orbital diagrams orbital diagrams help visualize which orbitals the electrons. Discover the three rules for creating electron orbital charts, and study examples of filling electron orbital in a diagram. Web a) draw the molecular orbital diagram of the given compounds, predict the bond order, and comment on whether the molecule can exist or not. Carbon (atomic number 6) has six electrons. Define bond order, and state its significance. This makes. The shape of s orbitals Define bond order, and state its significance. Web describe the essential difference between a sigma and a pi molecular orbital. Web orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy to both create and interpret. Web text 5 orbital. Below are dot density diagrams, boundary surface diagrams, and a rotating image. Web text 5 orbital diagrams orbital diagrams help visualize which orbitals the electrons in an atom are the basics of orbital diagrams there are different types of orbitals, that all have different energy levels. Individual atomic orbitals (ao) are arranged on the far left and far right of. Let us represent the shapes of orbitals with the help of boundary surface diagrams: Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. #1 find electrons of fluorine. Web orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. Web general notes on molecular orbital. The aufbau principle states that electrons occupy atomic orbitals in an ascending energy. As per hund’s rule, the degenerate atomic orbitals are first singly filled and then pairing occurs. Be 2 s, f 2 , cn − o) is cn − diamagnetic or paramagnetic? This makes it a challenge to draw, but i will show you the. Valence bond theory. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. Web an orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. Four of them fill the 1s and 2s orbitals. The electron configuration. #1 find electrons of fluorine. Construct a molecular orbital diagram of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. A box, line, or circle, is drawn to represent each orbital in the electron configuration. Web this chemistry video tutorial provides a basic introduction. We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. Web general notes on molecular orbital diagrams. Once the atomic number has been identified, write the. Web orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. Web an orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. (using the aufau principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. Web a p orbital which extends along the x axis is labeled a p x orbital. Web this wave function also helps us in drawing boundary surface diagrams. As per hund’s rule, the degenerate atomic orbitals are first singly filled and then pairing occurs. Web learn how to draw orbital diagrams. The aufbau principle states that electrons occupy atomic orbitals in an ascending energy. Web write the electron configuration for phosphorus and draw the orbital diagram. Valence bond theory (vbt) in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. Discover the three rules for creating electron orbital charts, and study examples of filling electron orbital in a diagram. Next, moving from the bottom up, we will draw the sublevels for each principal energy level. You can effortlessly find every single detail about the elements from this single interactive periodic table.
Drawing Orbital Diagrams

Molecular Orbital Diagrams simplified by Megan Lim Medium

Orbital Diagrams — Overview & Examples Expii

6.6 3D Representation of Orbitals Chemistry LibreTexts
![Distribution of Electrons in Different Orbits [with Examples] Teacho](https://d77da31580fbc8944c00-52b01ccbcfe56047120eec75d9cb2cbd.ssl.cf6.rackcdn.com/00d8e8eb-2904-4147-abf9-6d87a6c24f05/14.-orbits-teachoo-01.png)
Distribution of Electrons in Different Orbits [with Examples] Teacho

Shapes of Atomic Orbitals — Overview & Examples Expii
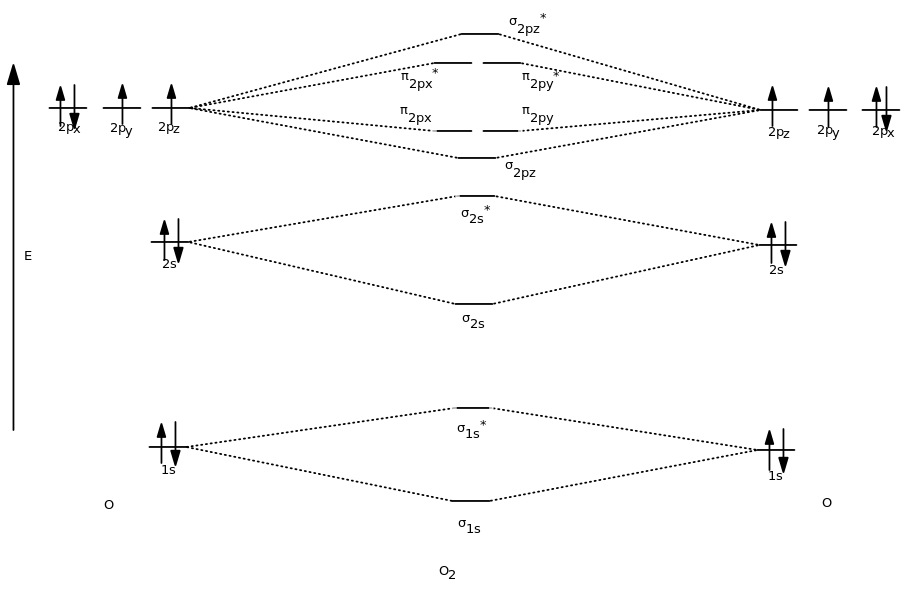
Drawing Atomic and Molecular Orbitals Diagrams for Molecules Organic

8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry

Top Notch Tips About How To Draw Orbital Diagrams Spellquestion

Atomic orbitals explained polizhuge
Compare The Bond Order To That Seen In The Lewis Structure (Remember That An Electron In An Antibonding Orbital Cancels The Stabilization Due To Bonding Of An Electron In A Bonding Orbital).
Construct A Qualitative Molecular Orbital Diagram For Chlorine, Cl 2.
Carbon (Atomic Number 6) Has Six Electrons.
Web Text 5 Orbital Diagrams Orbital Diagrams Help Visualize Which Orbitals The Electrons In An Atom Are The Basics Of Orbital Diagrams There Are Different Types Of Orbitals, That All Have Different Energy Levels.
Related Post: