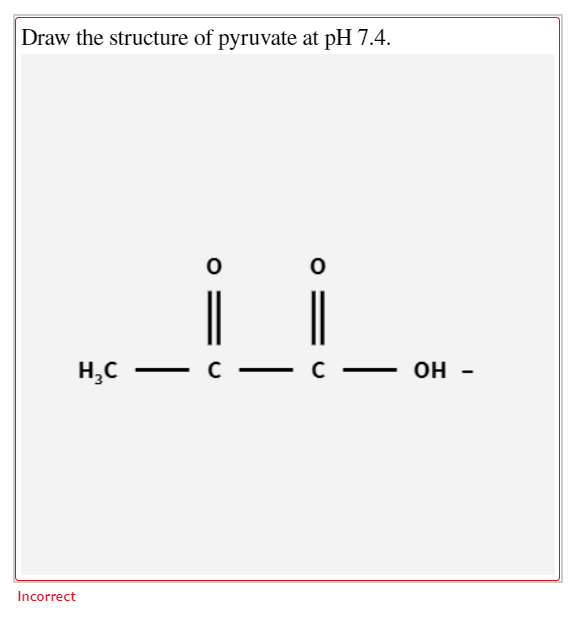
Draw The Structure Of Pyruvate At Ph 7.4
Draw The Structure Of Pyruvate At Ph 7.4 - Draw the structure and titration curve of cysteine by hand. 22 > select the structure of pyruvate showing its appropriate structure at ph 7.4. (only the nicotinamide ring needs to be drawn) click card to see definition 👆 draw click again to see term 👆 1/23 previous ← next Web add coefficients to the reaction summary to show the net results of glycolysis. W the structure of pyruvate. Molecular formula c 3 h 3 ko 3; Web 23.3 pyruvate oxidation and the citric acid cycle. Web add coefficients to the reaction summary to show the net results of glycolysis. Glucose + a adp + b p; Web chemistry chemistry questions and answers 48·the structure of pyruvic acid is shown below. Web select the structure of pyruvate showing its appropriate structure at ph 7.4. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Glucose + a adp + b p; You do not need to add the water and hydrogen ions necessary to balance the overall reaction. + c nad → x pyruvate +. Molecular weight 87.05 g/mol computed by pubchem 2.1 (pubchem release 2021.05.07) parent compound cid 1060 (pyruvic acid) dates create: Glucose + a adp + b p; W the structure of pyruvate. It is a conjugate acid of a pyruvate. Instead, it will exist as pyruvate (ch3cocoo⁻), which is a negatively charged ion. Web given the structure of pyruvate below, draw the reaction with nadh to form lactate. Web at a ph of 7. Web p k a pk_a p k a for pyruvic acid is 2.50 and since this value is lower than the given p h ph p h value (p h = 7.4 ph=7.4 p h = 7.4), we can. Web many important biochemicals are organic acids, such as pyruvic acid (pka = 2.50) and lactic acid (pka = 3.86). It is a conjugate acid of a pyruvate. They will be carried by nadh and fadh 2 to the complexes of the electron transport chain. Draw the structure of pyruvate, showing its appropriate structure at ph 7.4. 9780073511177 (2 more). Solution verified answered 3 months ago create an account to view solutions by signing up, you accept quizlet's recommended textbook solutions chemistry: (only the nicotinamide ring needs to be drawn) click card to see definition 👆 draw click again to see term 👆 1/23 previous ← next Web draw pyruvate as it would occur at the ph of most body. It has a role as a fundamental metabolite and a cofactor. Draw the structure and titration curve of cysteine by hand. + c nad → x pyruvate + y atp + z nadh you do not need to add the water and hydrogen ions necessary to balance the overall reaction. Glucose + a adp + b p: This problem has. They will be carried by nadh and fadh 2 to the complexes of the electron transport chain. Instead, it will exist as pyruvate (ch3cocoo⁻), which is a negatively charged ion. Assuming that the concentrations of each reactant and product are 1 m and that the reaction is performed at ph 7 and 25 °c, determine the δ𝐸′°δe′° for the reaction.. Each of its six carbons has been released as carbon dioxide, and all of the high energy electrons that can be harvested have been. Solution verified answered 3 months ago create an account to view solutions by signing up, you accept quizlet's recommended textbook solutions chemistry: Web select the structure of pyruvate showing its appropriate structure at ph 7.4. Based. Draw the structure and titration curve of cysteine by hand. + c nad+ + x pyruvate + y atp + z nadh you do not need to add the water and hydrogen ions necessary to balance the overall reaction. Web add coefficients to the reaction summary to show the net results of glycolysis. In the presence of oxygen, pyruvate is. Names properties searches spectra articles crystal cifs more names and synonyms database id (s) Web draw the structure of pyruvate, showing its appropriate structure at ph 7.4. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web chemistry chemistry questions and answers draw the structure of pyruvate, showing its appropriate structure at ph. Glucose + a adp + b p: A = b= c= x= y = z = draw the structure of pyruvate at ph 7.4. + c nad → x pyruvate + y atp + z nadh you do not need to add the water and hydrogen ions necessary to balance the overall reaction. Ra (b) using what you have learned about acidic functional groups, which form of this compound is likely to predominate in the cell at ph 7.4? Molecular formula c 3 h 3 nao 3; It is functionally related to a propionic acid. Web draw the structure of pyruvate, showing its appropriate structure at ph 7.4. 9780073511177 (2 more) patricia amateis, silberberg 6,032 solutions Web at a ph of 7. The resulting acetyl coa can enter several pathways, but most often, the acetyl group is delivered to the citric acid cycle for further catabolism. It is a conjugate acid of a pyruvate. Web add coefficients to the reaction summary to show the net results of glycolysis. In the presence of oxygen, pyruvate is transformed into an acetyl group attached to a carrier molecule of coenzyme a. Web add coefficients to the reaction summary to show the net results of glycolysis. (only the nicotinamide ring needs to be drawn) click card to see definition 👆 draw click again to see term 👆 1/23 previous ← next Glucose + a adp + b p;draw the structure of pyruvate at ph 7.4. metalwallartphotography

Pyruvate Metabolism Biochemistry Medbullets Step 1
Draw the Structure of Pyruvate at Ph 7.4 TrendingWorld
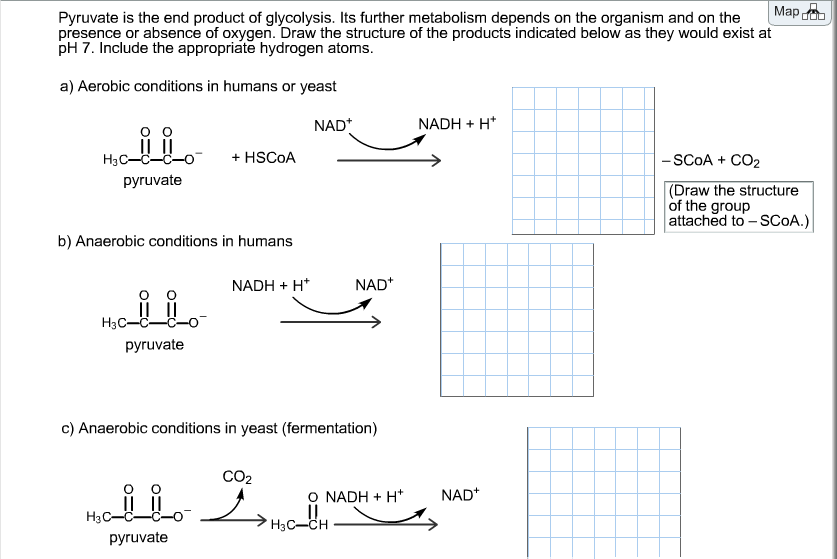
draw the structure of pyruvate at ph 7.4. lesbianlineartdrawingsimple

draw the structure of pyruvate at ph 7.4. vangalderjanesvillewi

Pryruvate Oxidation And The Krebs Cycle ALevel Biology Revision Notes

Simplistic illustration of the pyruvate cycle showing pyruvate

Pyruvate Definition, Structure & Uses Video & Lesson Transcript
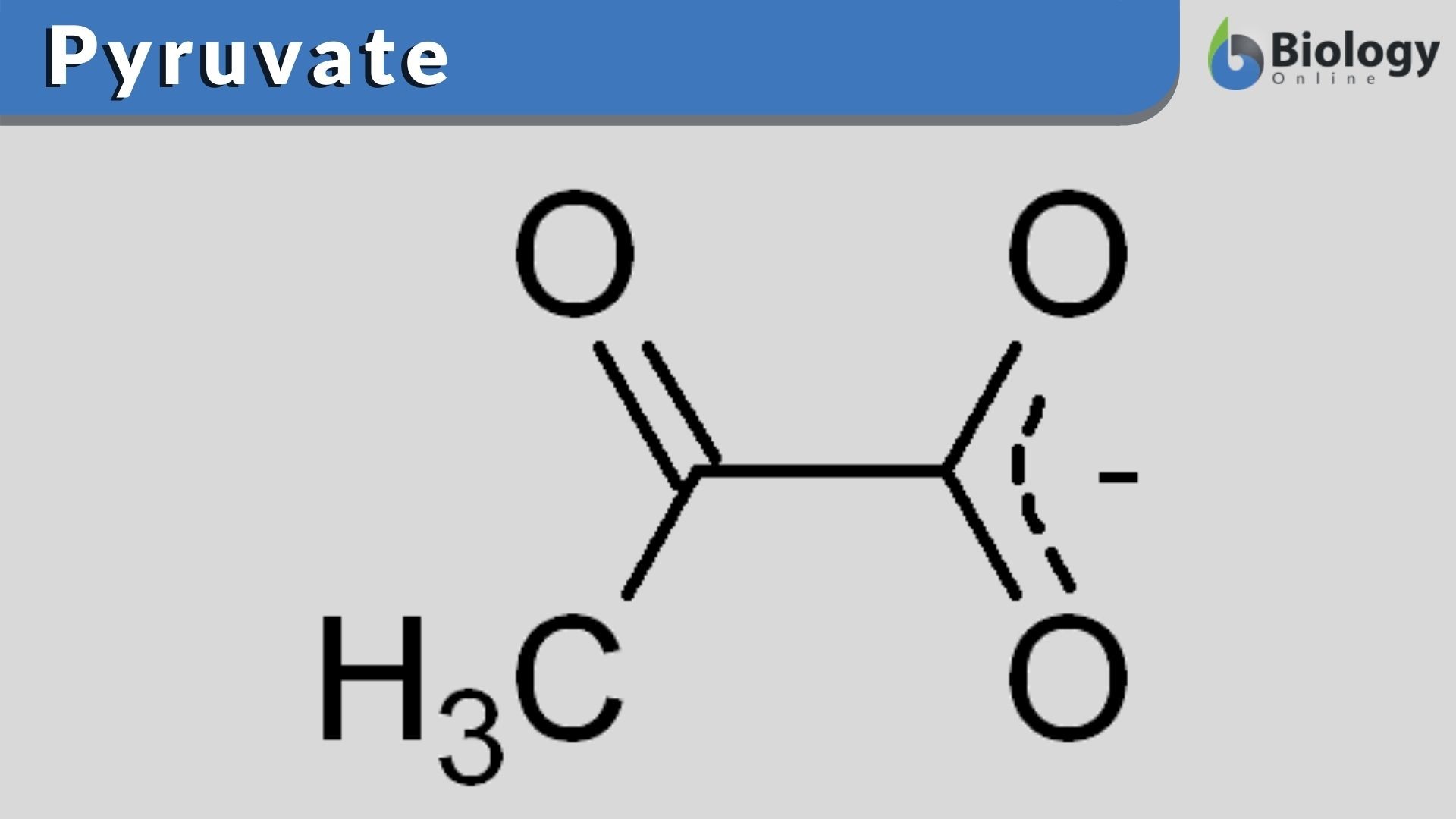
Pyruvate Definition and Examples Biology Online Dictionary
:max_bytes(150000):strip_icc()/pyruvate-42bb6b4e0b01439ab406db94e5e9fafa.jpg)
Pyruvate Facts and Oxidation
+ C Nad+ + X Pyruvate + Y Atp + Z Nadh You Do Not Need To Add The Water And Hydrogen Ions Necessary To Balance The Overall Reaction.
Then, Draw The Structure Of Pyruvate At Ph 7.4.
Solution Verified Answered 3 Months Ago Create An Account To View Solutions By Signing Up, You Accept Quizlet's Recommended Textbook Solutions Chemistry:
This Problem Has Been Solved!
Related Post: