Drawing Of A Hydrogen Atom
Drawing Of A Hydrogen Atom - The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0, m s = + 1 2 m s = + 1 2). Web draw tower grating (dtg) with large capacity, long distance, fast response and other advantages is rapidly becoming the current mainstream fiber optic sensors, but want to write high reflectivity fiber on the draw tower fiber is particularly difficult. Atom labels for all other elements are shown. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the h atom. Web drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. Check how the prediction of the model matches the experimental results. Thomson, the model suggested that the atom was a spherical ball of positive charge, with negatively charged electrons scattered evenly throughout. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. Web summarize how bohr’s quantum model of the hydrogen atom explains the radiation spectrum of atomic hydrogen historically, bohr’s model of the hydrogen atom is the very first model of atomic structure that correctly explained. Web following hydrogen is the noble gas helium, which has an atomic number of 2. Web drawing skeletal or line diagrams how do you convert a structural representation, showing all the atoms and bonds, into a skeletal diagram? Thus the most probable radius obtained from quantum mechanics is identical to the radius calculated by classical mechanics. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the h atom.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. The second electron also goes into the 1s orbital and fills that orbital. Web solving schrödinger’s equation for hydrogen. It also misses out all the carbon atoms. Also, helium is shown in group 8a, but it only has. Web proposed in 1904 by j. U(r, θ, ϕ) = − ke2 r. Thomson, the model suggested that the atom was a spherical ball of positive charge, with negatively charged electrons scattered evenly throughout. Top voted ali arshad 9 years ago what does the negative sign in energy term represent ? Electron shells consist of one or more subshells, and. Atom labels for all other elements are shown. Web a hydrogen atom is an atom of the chemical element hydrogen. Identify the physical significance of each of the quantum numbers (n, l, m) of the hydrogen atom; Web describe the hydrogen atom in terms of wave function, probability density, total energy, and orbital angular momentum; The schrödinger wave equation for. A skeletal representation ignores the hydrogen atoms and their bonds to carbon. Web for the hydrogen atom, the peak in the radial probability plot occurs at r = 0.529 å (52.9 pm), which is exactly the radius calculated by bohr for the n = 1 orbit. Web drawing skeletal or line diagrams how do you convert a structural representation, showing. And so you see it forms this tetrahedral shape, it's pretty close to a tetrahedron. And then you have the covalent bond to the other hydrogen atom. Questions tips & thanks want to join the conversation? Atom labels for all other elements are shown. Web hydrogen atoms are omitted but are assumed to be present to complete each of carbon's. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Another lone pair of electrons back here. U(r, θ, ϕ) = − ke2 r. Web drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite. The schrödinger wave equation for the hydrogen atom. Check how the prediction of the model matches the experimental results. Top voted ali arshad 9 years ago what does the negative sign in energy term represent ? The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0,. The diameter of a hydrogen atom is roughly 100,000 times larger than a proton. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the h atom. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. A skeletal. For hydrogen, the potential energy function is simply: The helium atom contains two protons and two electrons. Web following hydrogen is the noble gas helium, which has an atomic number of 2. Top voted ali arshad 9 years ago what does the negative sign in energy term represent ? The electrically neutral atom contains a single positively charged proton and. Hydrogens that are attached to elements other than carbon are shown. Web hydrogen atoms are omitted but are assumed to be present to complete each of carbon's four bonds. Also, helium is shown in group 8a, but it only has two valence electrons. Therefore, if we make a proton the size of the picture above, 1000 pixels across, then the electron orbiting this proton is located 50,000,000 pixels to the right (but could be found anywhere in the sphere around the. The helium atom contains two protons and two electrons. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. Web i'll draw it as a little green circle there. A very simple drawing of an hydrogen atom with a nucleus and an electron :).more. And then you have the covalent bond to the other hydrogen atom. For hydrogen, the potential energy function is simply: Thomson, the model suggested that the atom was a spherical ball of positive charge, with negatively charged electrons scattered evenly throughout. Web the electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Try out different models by shooting light at the atom. And so you see it forms this tetrahedral shape, it's pretty close to a tetrahedron. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the h atom. Top voted ali arshad 9 years ago what does the negative sign in energy term represent ?
Hydrogen Definition, Structure, Properties, and Uses
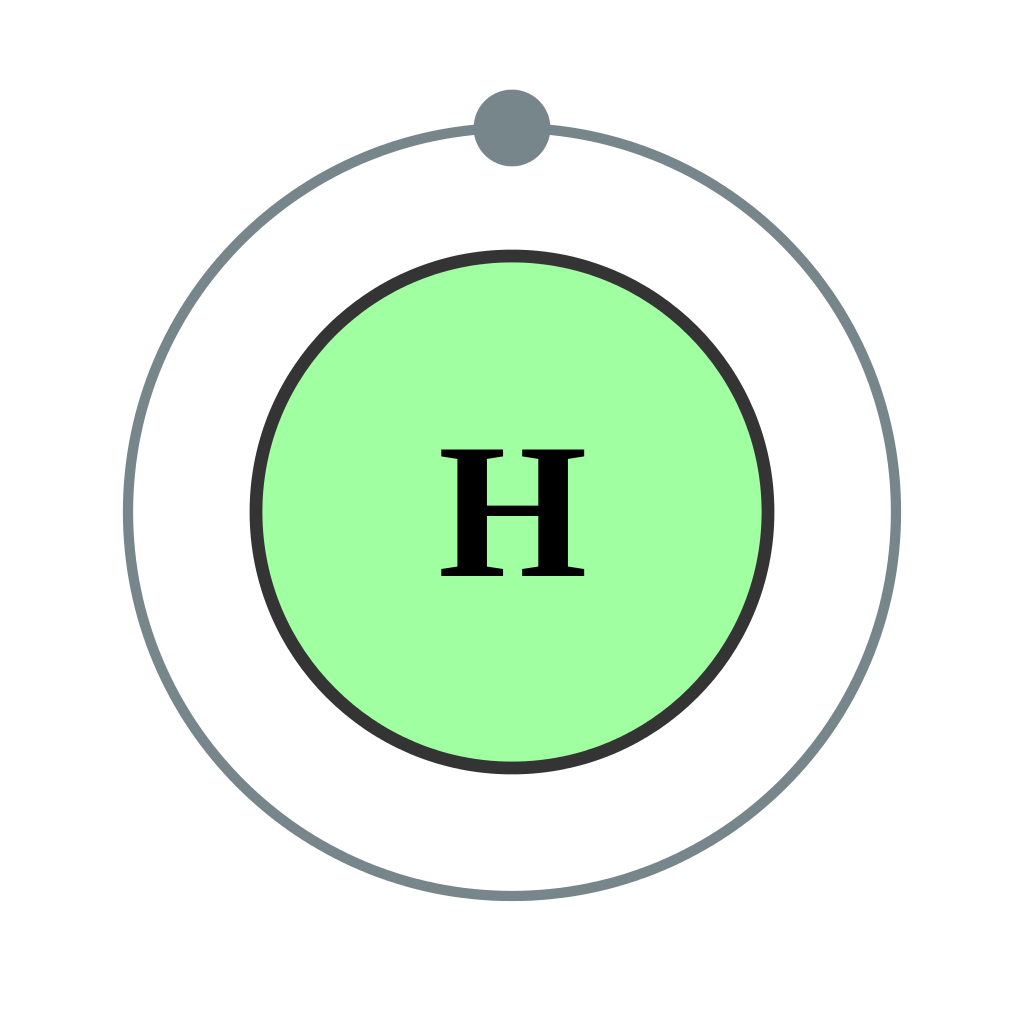
FileElectron shell 001 Hydrogen no label.svg Wikipedia

Describe Bohr’s model of the hydrogen atom. bitWise Academy

Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy

Bohr Atomic Model Of Hydrogen

Diagram representation element hydrogen Royalty Free Vector
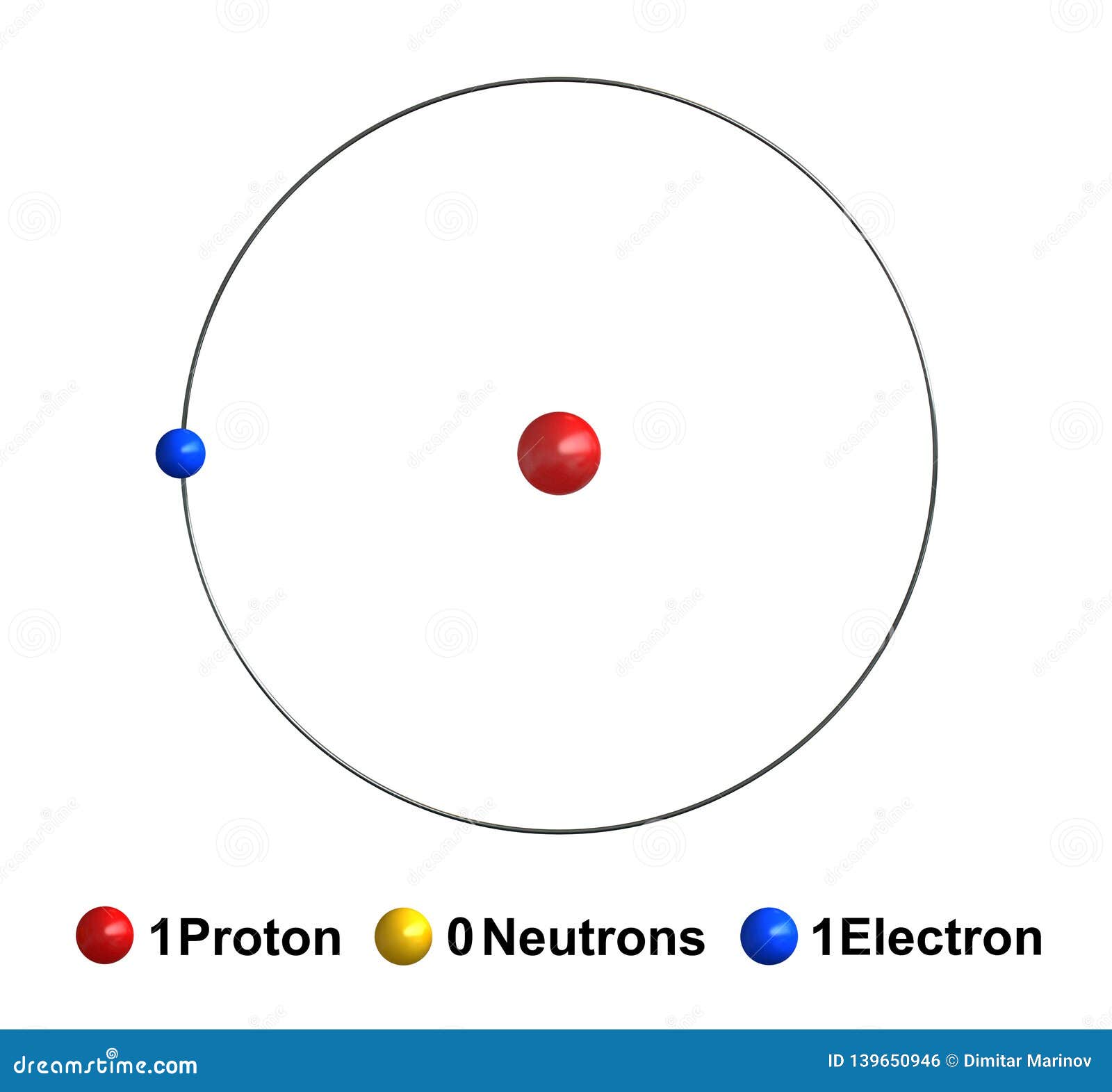
Hydrogen stock illustration. Illustration of education 139650946

Hydrogen atom on white background Royalty Free Vector Image

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps

Diagram Representation Of The Element Hydrogen Stock Vector Image
Web Describe The Hydrogen Atom In Terms Of Wave Function, Probability Density, Total Energy, And Orbital Angular Momentum;
Then You Have The Covalent Bond.
U(R, Θ, Φ) = − Ke2 R.
Web Proposed In 1904 By J.
Related Post: