How To Draw A Atomic Structure
How To Draw A Atomic Structure - Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Hydrogen has 1 proton and no neutrons. Web an isotope of any element can be uniquely represented as \(^a_z x\), where x is the atomic symbol of the element. Advances in nuclear and subatomic physics. The isotope of carbon that has 6 neutrons is therefore \(_6^{12} c\). The negatively charged particles called electrons revolve around the centre of the nucleus. Web models of atomic structure. Label them with their charge. All atom labels are shown and all lone pairs are shown. The element which exist in different forms, having same atomic number but different mass number. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. The negatively charged particles called electrons revolve around the centre of the nucleus. All atom labels are shown and all lone pairs are shown. Draw three. Web let's learn how to draw an atomic structure drawing atomic structure or diagram is quite easy and i am sure you will be able to draw it as well. Web how to draw an an atomic structure? Web having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how. Web 2.8m views 5 years ago. Quantum field theory and the standard model Hydrogen has 1 proton and no neutrons. The electron shells are shown, moving outward from the nucleus. Web atomic basics answer key part a: The center of an atom is the nucleus and one or more electrons surrounding the nucleus. The mass number of fluorine is 19 and atomic number is 9. Web an isotope of any element can be uniquely represented as \(^a_z x\), where x is the atomic symbol of the element. Protons, neutrons, and photons a protons, neutrons, and photons positrons,.. Draw three electrons in the second energy level and label them with their charge. Add enough electrons (dots) to the outer atoms to. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass. Quantum field theory and the standard model Web models of atomic structure. The element which exist in different forms, having same atomic number but different mass number. Web student note:this video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from periodic table. Web an isotope of any element can be uniquely represented as \(^a_z x\), where x is the atomic. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Add enough electrons (dots) to the outer atoms to. The number of dashes indicate whether the bond is a single, double, or triple covalent bond. The structure of the atom. Then play a game to test your ideas! Draw six neutrons in the nucleus of the atom. Web atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Advances in nuclear and subatomic physics. Web simple rules to guide you to draw atomic structures! Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. The laws of quantum mechanics; Web how to draw an an atomic structure? This allows us to determine which orbitals are occupied by electrons in each atom. Draw six neutrons in the nucleus of the atom. The number of dashes indicate whether the bond is a single, double, or triple covalent bond. All atom labels are shown and all lone pairs are shown. Hydrogen has 1 proton and no neutrons. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The subscript indicating the atomic number is actually redundant because the atomic symbol already uniquely specifies z. The negatively charged particles called electrons revolve around. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. Covalent bonds are shown using lines. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Draw six neutrons in the nucleus of the atom. Web 0:00 / 3:02 drawing atomic structures schoolrevision 58 subscribers subscribe 120 share save 19k views 9 years ago in this video i have used the example of sodium,. Drawing atomic structure requires only a simple understanding of the components of atomic structure. Web 2.8m views 5 years ago. Web simple rules to guide you to draw atomic structures! Draw five protons in the nucleus of the atom. This allows us to determine which orbitals are occupied by electrons in each atom. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. A detailed explanation of drawing an atomic structure while following the rules. Web an isotope of any element can be uniquely represented as \(^a_z x\), where x is the atomic symbol of the element. Hydrogen has 1 proton and no neutrons. Web let's learn how to draw an atomic structure drawing atomic structure or diagram is quite easy and i am sure you will be able to draw it as well. Web models of atomic structure.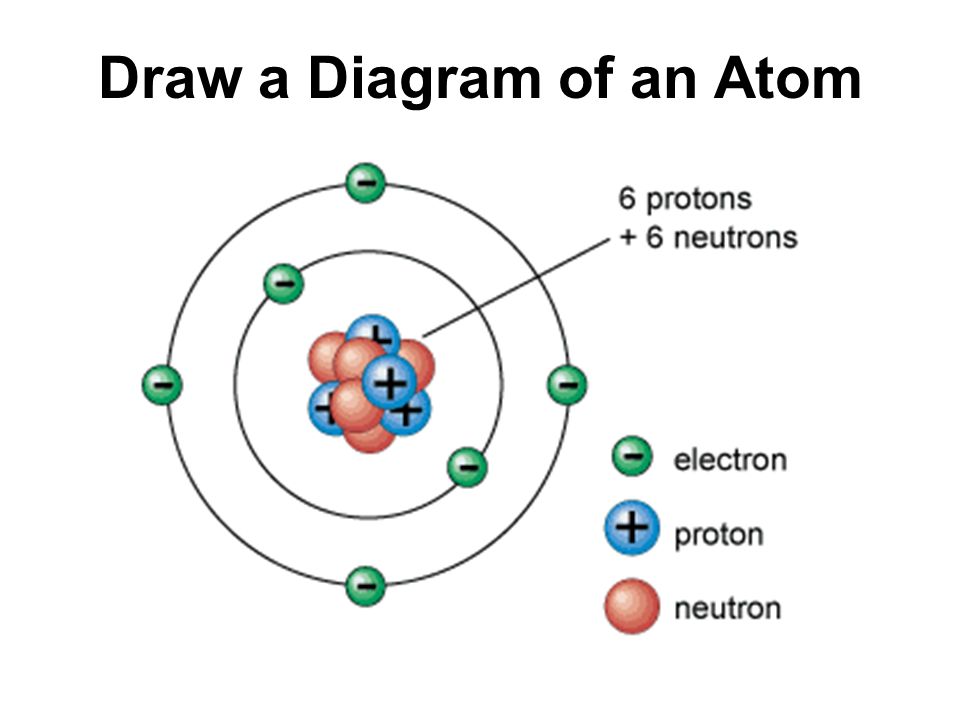
Skills Practice AMAZING 8TH GRADE SCIENTISTS

How To Draw An Atom, Step by Step, Drawing Guide, by Dawn DragoArt
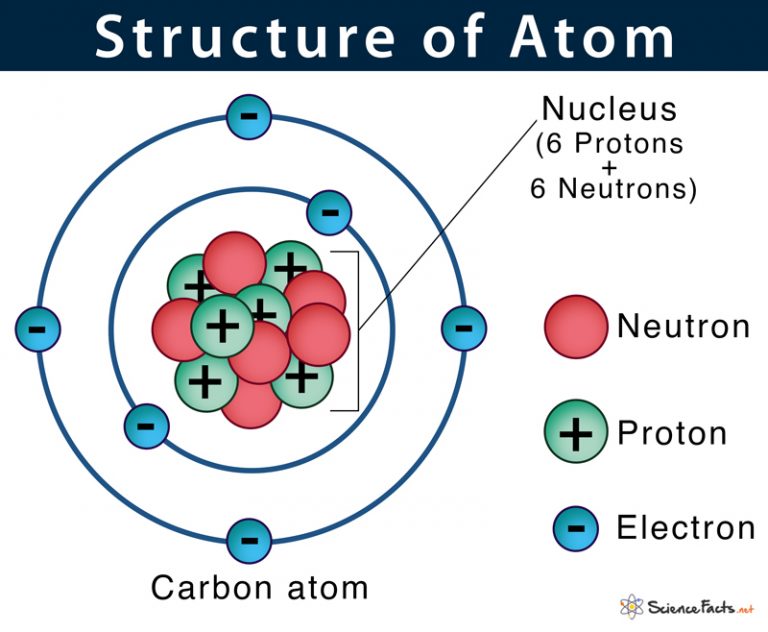
Atom Definition, Structure & Parts with Labeled Diagram

Atom Definition, Structure & Parts with Labeled Diagram

Drawing Atoms Montessori Muddle
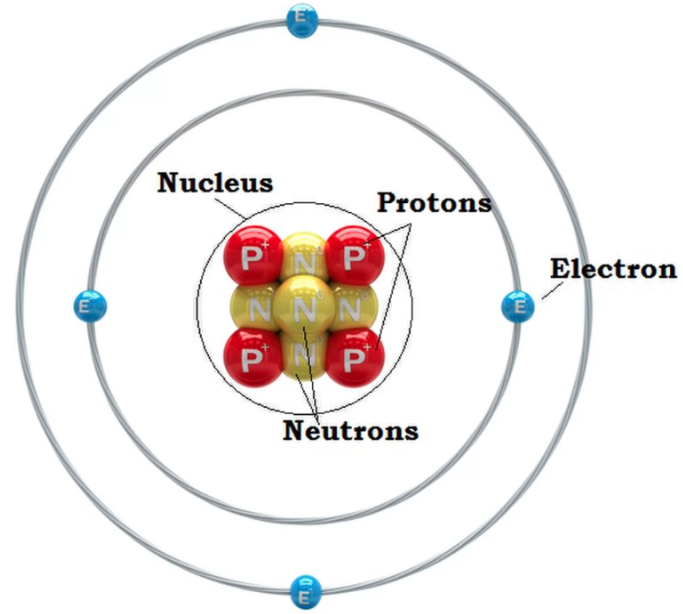
The Atom Mumley Science
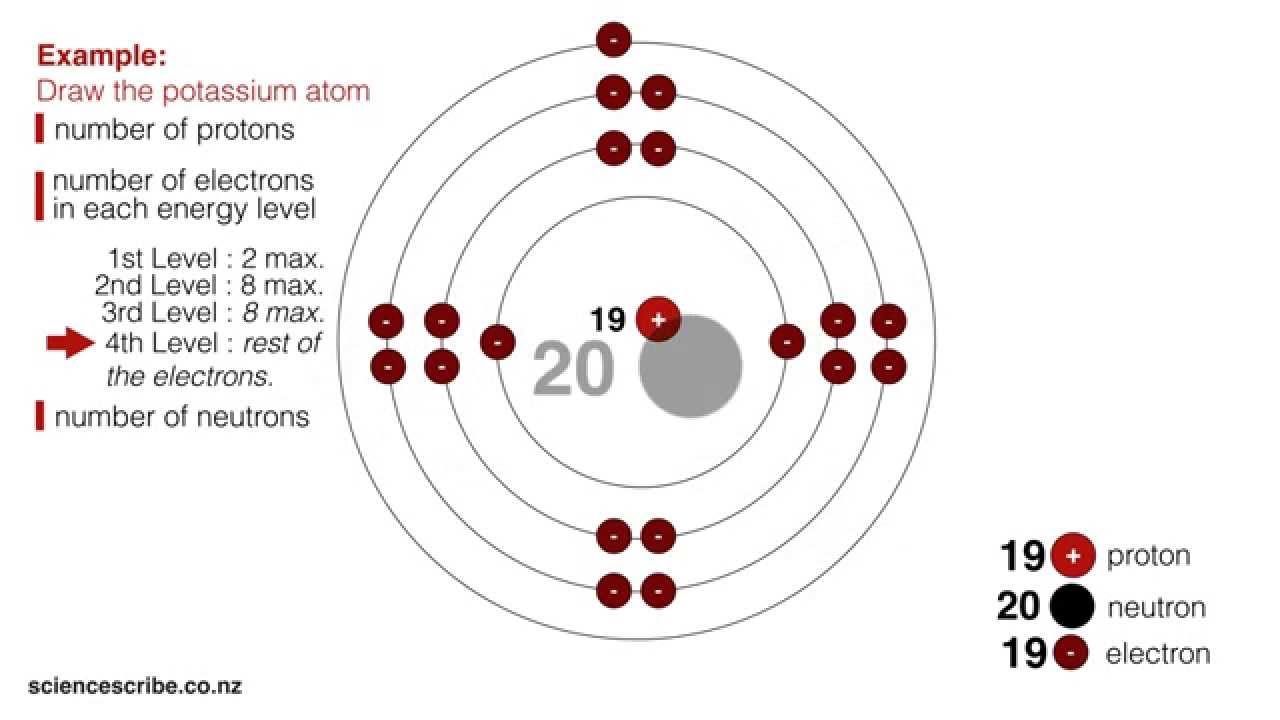
Drawing Atoms (NCEA L1 & Junior Science) YouTube
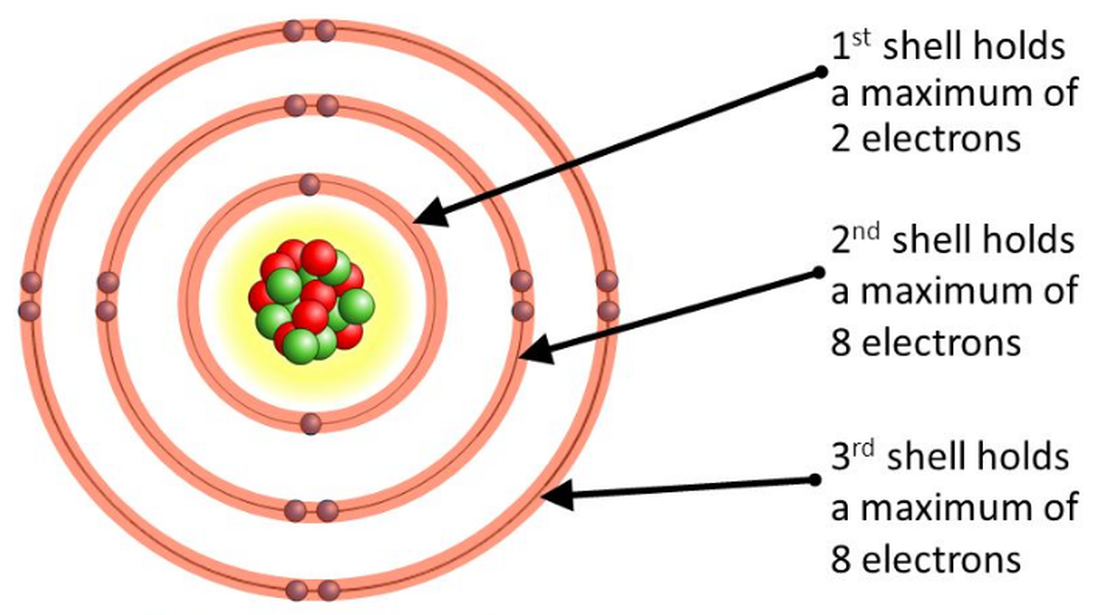
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons

How to draw Atom structure diagram step by step l Atomic structure
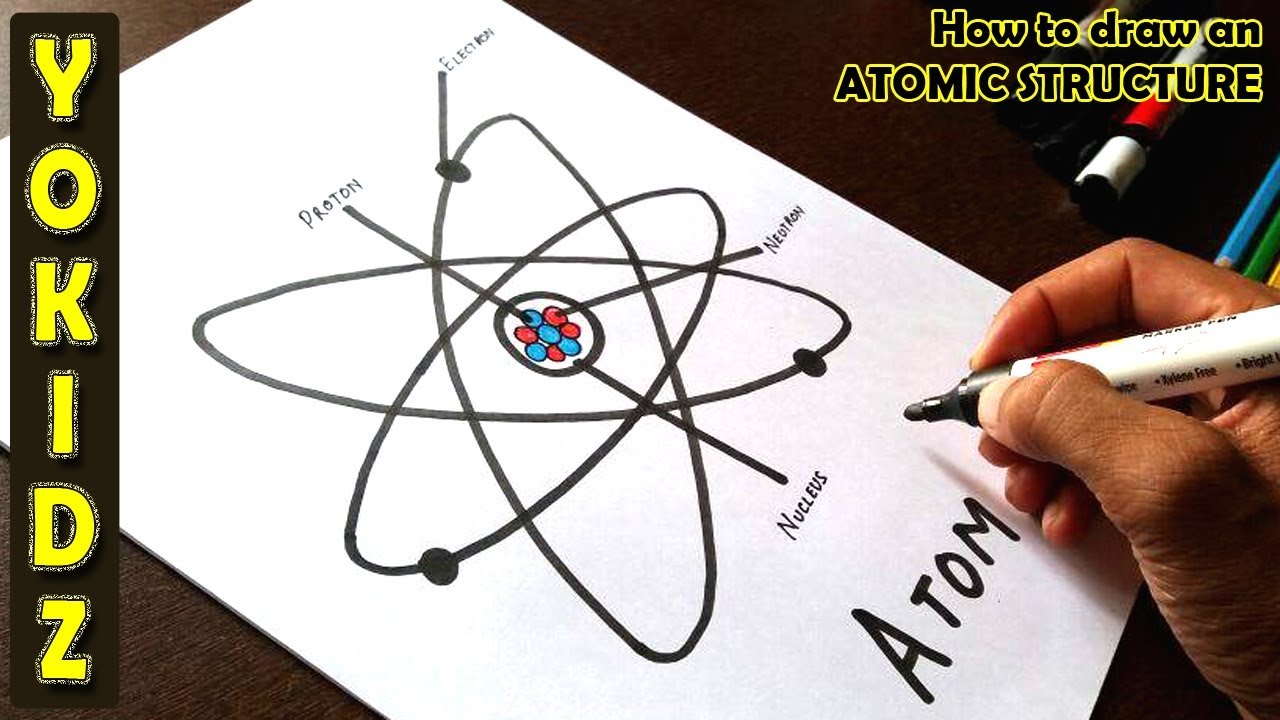
How to draw an ATOMIC structure YouTube
Web Basic Diagram Of An Atom.
Protons, Neutrons, And Photons A Protons, Neutrons, And Photons Positrons,.
The Mass Number Of Fluorine Is 19 And Atomic Number Is 9.
Most Of An Atom Is Just Empty Space And Consists Of A Positively Charged Nucleus Of Protons And Neutrons Surrounded By A Cloud Of Negatively Charged Electrons.
Related Post: