How To Draw Oxygen
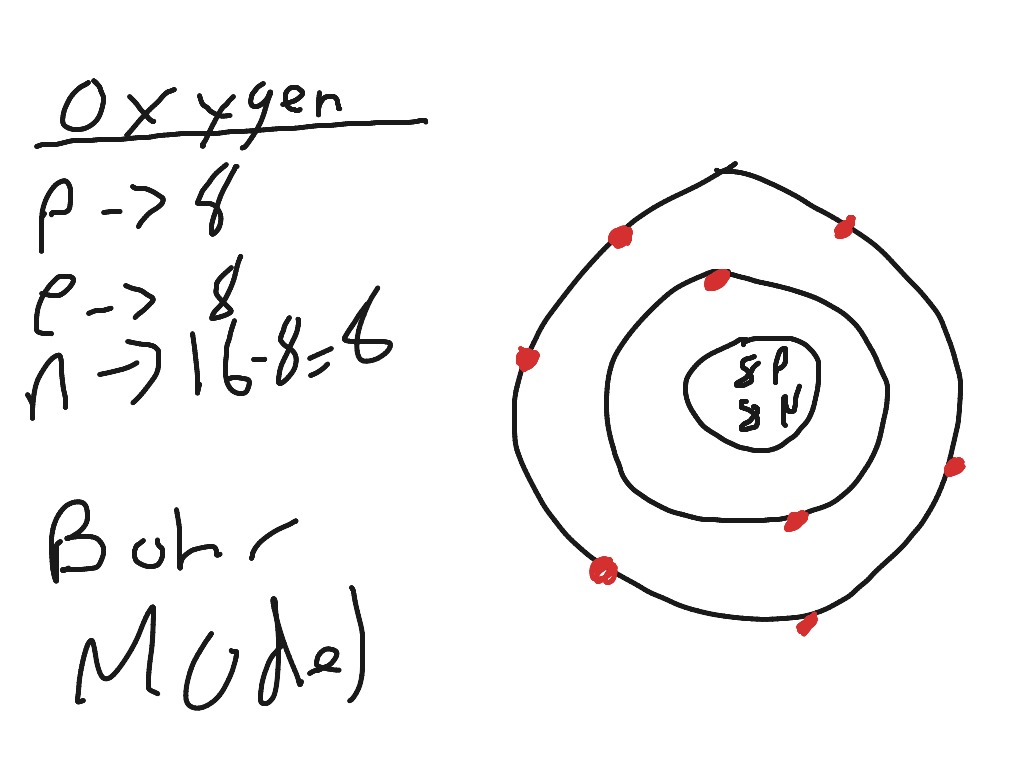
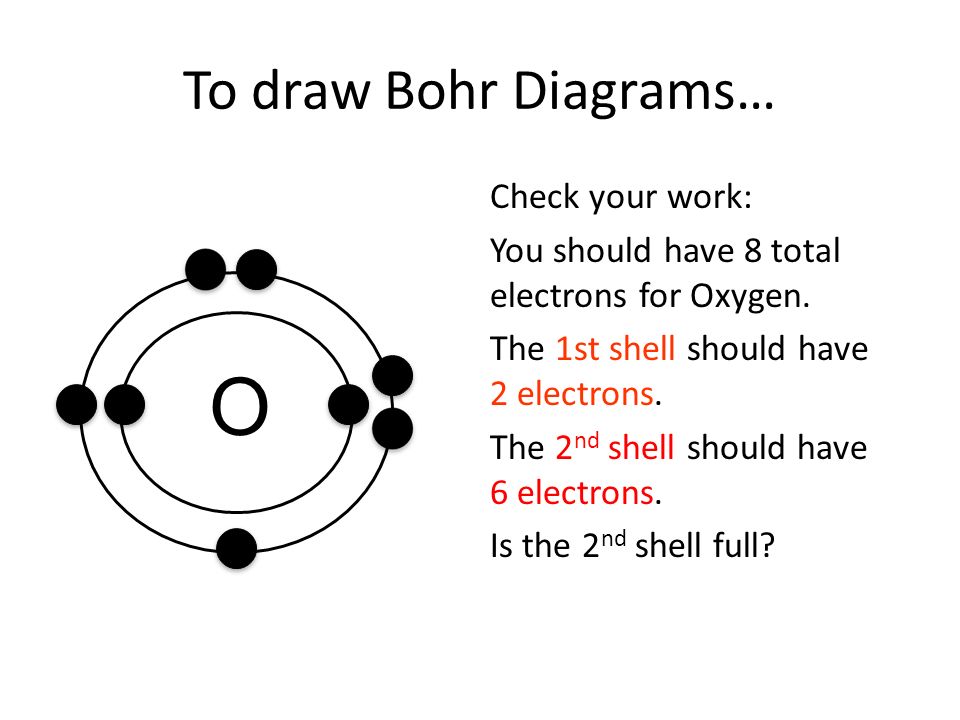
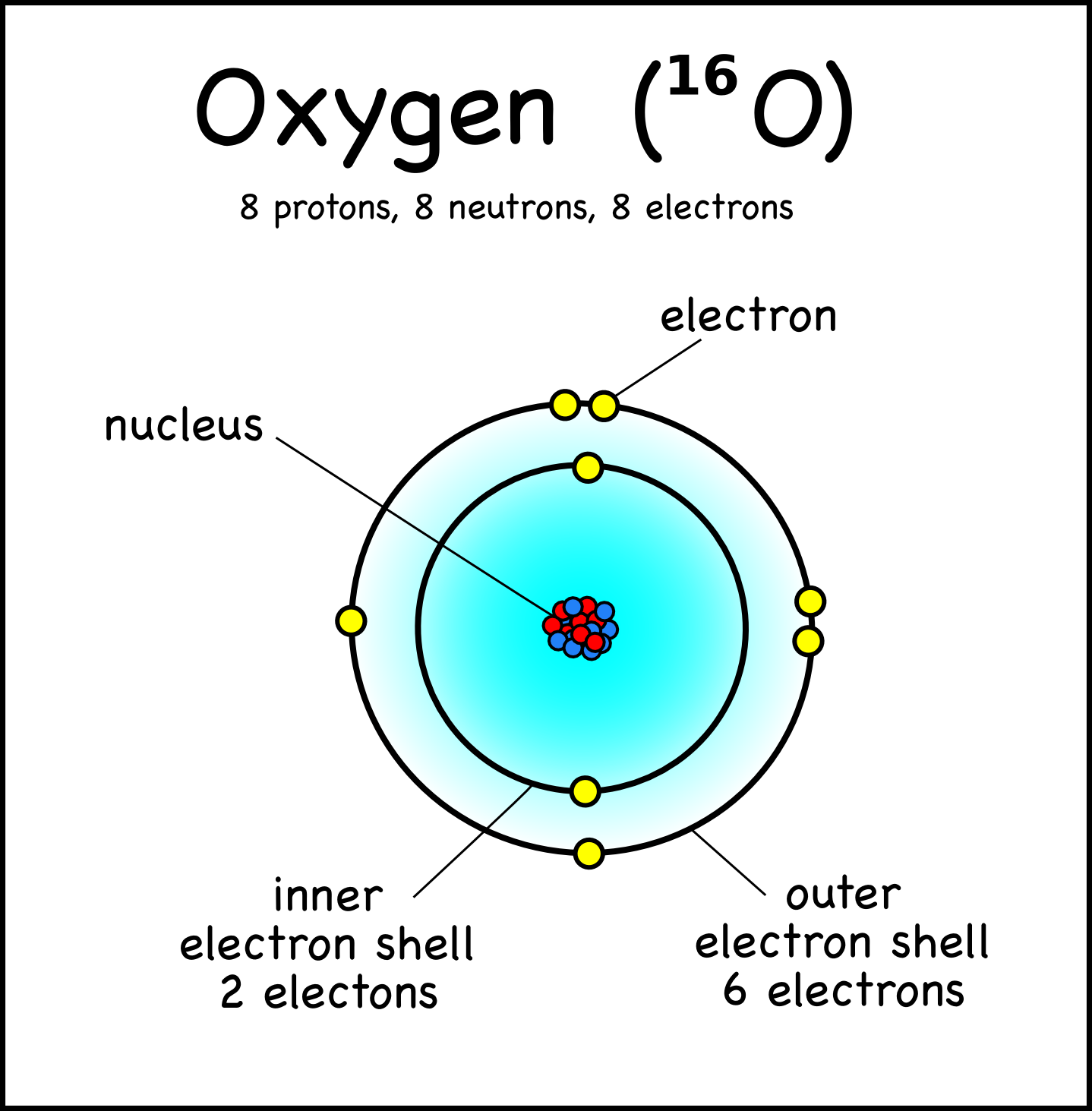
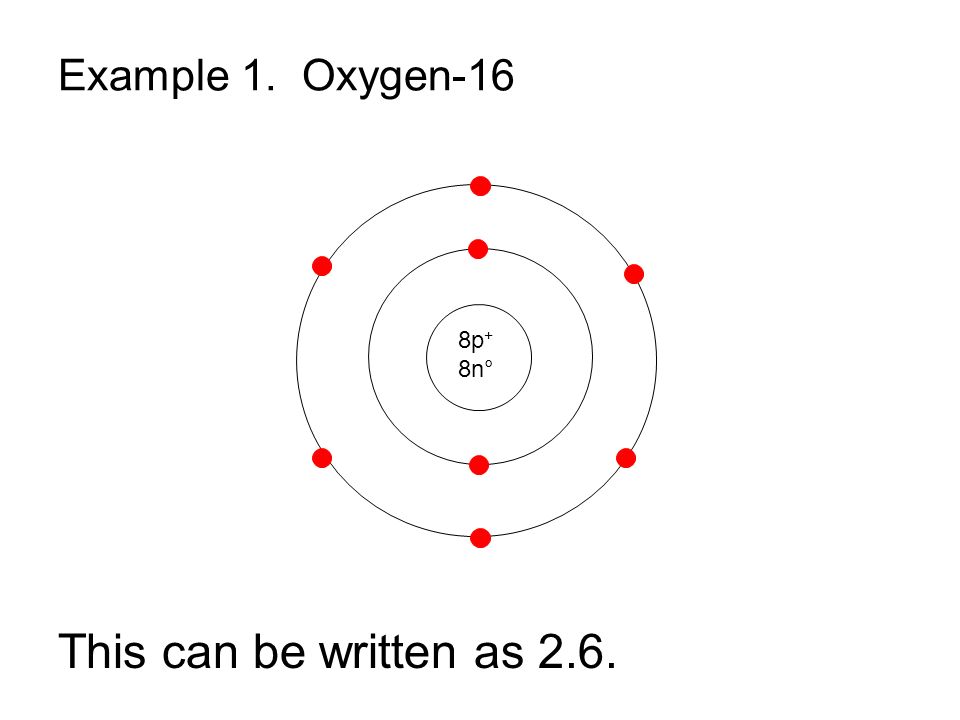
How To Draw Oxygen - The shell closest to the nucleus is. Web the first step is to sketch the lewis structure of the o2 molecule, to add valence electrons around the two oxygen atoms, and the final step is to combine the two oxygen diatomic atoms to get the o2 lewis structure. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on. It occurs as a diatomic gas and is also the third most abundant element in the universe. Figure 2 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Web hi, welcome to athaya drawing!thanks for watching, like, share, comment, and subscribe.how to draw oxygen tank#oxygentank #oxygen In order to draw the lewis structure of o2, first of all you have to find the total number of valence electrons present in the o2 molecule. Web 6 steps to draw the lewis structure of o2 step #1: Therefore, the oxygen atom in methanol owns 2 + 2 + (½ x 4) = 6 valence electrons. It is a colorless and odorless gas with oxidizing. Add enough electrons (dots) to the outer atoms to. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. How to draw an oxygen mask. Considers electrons delocalized throughout the entire molecule. The shell closest to the nucleus is. Web hi, welcome to athaya drawing!thanks for watching, like, share, comment, and subscribe.how to draw oxygen tank#oxygentank #oxygen Therefore, it is important to further study the grating formation mechanism of fiber gratings for the rapid. An oxygen atom has 8 protons and electrons. Web step 1: Web there are two main ways to measure or test blood oxygen levels: A blood draw test provides much more information about your oxygen levels than an oximeter does. In order to draw the lewis structure of o2, first of all you have to find the total number of valence electrons present in the o2 molecule. Considers bonds as localized between one pair of atoms. I show you where oxygen is on the. Web draw tower grating (dtg) with large capacity, long distance, fast response and other advantages is rapidly becoming the current mainstream fiber optic sensors, but want to write high reflectivity fiber on the draw tower fiber is particularly difficult. Draw the dot and cross diagram with the outer shells overlapping. Web there are two main ways to measure or test. Web 79 views 2 years ago. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) oxygen (o) electron configuration (bohr model) electron configuration through orbitals follows different principles. Web if we look at the acetate anion, so we just talked about the fact that one of these lone pairs here, so this is not localized to the. Here, the given molecule is o2 (oxygen). Take a look at the diagram again. It occurs as a diatomic gas and is also the third most abundant element in the universe. Include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. A blood draw test provides much more information about your. Web nitroxyl contains a single covalent bond between hydrogen and nitrogen and a double covalent bond between nitrogen and oxygen. Through a blood draw test and through pulse oximetry (using an oximeter). Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. 16 th group of the periodic table.. Considers bonds as localized between one pair of atoms. Web the first step is to sketch the lewis structure of the o2 molecule, to add valence electrons around the two oxygen atoms, and the final step is to combine the two oxygen diatomic atoms to get the o2 lewis structure. Here, the given molecule is o2 (oxygen). Web draw the. So diagrammatically, its atomic structure will have 8 electrons (2 in the first shell and 6 in the second shell) placed on the electrons shells (rings). Web nitroxyl contains a single covalent bond between hydrogen and nitrogen and a double covalent bond between nitrogen and oxygen. Web if we look at the acetate anion, so we just talked about the. Therefore, it is important to further study the grating formation mechanism of fiber gratings for the rapid. Web if we look at the acetate anion, so we just talked about the fact that one of these lone pairs here, so this is not localized to the oxygen; It is a colorless and odorless gas with oxidizing. In what kind of. An oxygen atom has 8 protons and electrons. In each resonance expression, identify the type of resonance motion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. It occurs as a diatomic gas and is also the third most abundant element in the universe. Web if we look at the acetate anion, so we just talked about the fact that one of these lone pairs here, so this is not localized to the oxygen; Considers electrons delocalized throughout the entire molecule. Web draw the major resonance contributor of the structure below. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Web nitroxyl contains a single covalent bond between hydrogen and nitrogen and a double covalent bond between nitrogen and oxygen. It is a colorless and odorless gas with oxidizing. Web the first step is to sketch the lewis structure of the o2 molecule, to add valence electrons around the two oxygen atoms, and the final step is to combine the two oxygen diatomic atoms to get the o2 lewis structure. I show you where oxygen is on the periodic table and how to determine how many. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) oxygen (o) electron configuration (bohr model) electron configuration through orbitals follows different principles. Web hi, welcome to athaya drawing!thanks for watching, like, share, comment, and subscribe.how to draw oxygen tank#oxygentank #oxygen Figure 2 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.
How to draw dot and cross diagram of Oxygen molecule YouTube
Bohr Model Drawing Of Oxygen at Explore collection
Bohr Model Drawing Of Oxygen at Explore collection

How to Draw Oxygen Cylinder with Regulator & Oxygen Face Mask, Cute
Bohr Model Drawing Of Oxygen at GetDrawings Free download
Bohr Model Drawing Of Oxygen at Explore collection

Diagram representation of the element oxygen Vector Image
Atomic Model Of Oxygen ClipArt Best

Drawing the structure of the oxygen molecule Brainly.in

Bohr Model Drawing Of Oxygen at GetDrawings Free download
In Order To Draw The Lewis Structure Of O2, First Of All You Have To Find The Total Number Of Valence Electrons Present In The O2 Molecule.
In What Kind Of Orbitals Are The Two Lone Pairs On The Oxygen?
Web Electron Configuration Can Be Done In Two Ways.
The First Shell Of Oxygen Has 2 Electrons And The Outer Shell Or Valence Shell Of Oxygen Has 6 Electrons, Hence, The Number Of Valence Electrons In The Oxygen Atom Is 6.
Related Post: