P Draw The Lewis Dot Structure For P .
P Draw The Lewis Dot Structure For P . - For the p (ch3) structure use the periodic table to find the total number of valence electrons for the p (ch3) molecule. Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the lewis symbols for the elements of the third period of the periodic table. Web part b draw the lewis dot structure for p. Web drawing lewis dot structures and resonance structures. Don't forget to include any positive or negative charges when determining this. Each symbol represents the nucleus and the core electrons of the atom. 1 shows the lewis symbols for the elements of the third period of the periodic table. Dots show where electrons are around the atoms, and lines or pairs of dots show where covalent bonds connect the atoms. Lewis diagram of formaldehyde (ch₂o) worked example: Shared pairs of electrons are drawn as lines between atoms, while. Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: ⚛️ lewis structures of atoms. A lewis structure contains symbols for the elements in a molecule, connected by lines and surrounded by pairs of dots. The following procedure will give. Note, the sulfur is sp 2 hybridized giving it a trigonal planar geometry, and the unhybridized p orbital forms the pi bond with with overlap of the p orbitals of each. Draw the simple structure (skeleton structure) of the compound by connecting everything with single bonds only. 1 shows the lewis symbols for the elements of the third period of. Draw the simple structure (skeleton structure) of the compound by connecting everything with single bonds only. Lewis diagram of xenon difluoride (xef₂) exceptions to the octet rule. If the species is an ion, add or subtract electrons corresponding to the charge. Lewis diagram of formaldehyde (ch₂o) worked example: Web follow these simple steps to correctly draw a lewis dot structure: Follow these simple steps to draw lewis dot structures: As such, the electron dot diagram for carbon is as follows: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: ⚛️ lewis. Web interpreting lewis structures. Web follow these simple steps to correctly draw a lewis dot structure: Show the formal charges of all atoms in the correct structure. Web the electron dot (lewis) structure of phosphorus can be represented by placing 5 dots around the atomic symbol “p”. The following procedure will give you the correct lewis structure for any molecule. The number of dots equals the number of valence electrons in the atom. Web how to draw lewis structures. Web part b draw the lewis dot structure for p. As such, the electron dot diagram for carbon is as follows: Web how to draw the lewis dot diagram of p (phosphorus) chemistnate 259k subscribers subscribe subscribed 33k views 4 years. These dots are arranged to the right and left and above and below the symbol. Figure 7.9 lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Note, the sulfur is sp 2 hybridized giving it a trigonal planar geometry, and the unhybridized p orbital forms the pi bond with with. The number of dots equals the number of valence electrons in the atom. The following is a general procedure for drawing lewis structures. Write the formula of each compound using the chemical symbols of each element: Draw the lewis dot structure for p. Lewis diagram of formaldehyde (ch₂o) worked example: Web however, conventionally, we draw the dots for the two p electrons on different sides. Draw the simple structure (skeleton structure) of the compound by connecting everything with single bonds only. Stages to articulate the electron dot formula are stated beneath. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. It will also. The number of dots equals the number of valence electrons in the atom. Follow these simple steps to draw lewis dot structures: Shared pairs of electrons are drawn as lines between atoms, while. As such, the electron dot diagram for carbon is as follows: Electron dots are typically arranged in four pairs located on the four sides of the atomic. I show you where phosphorous is on the periodic table and how to determine how many valence electrons. Part draw the lewis dot structure for b. Draw the simple structure (skeleton structure) of the compound by connecting everything with single bonds only. Web thus you can not draw a lewis dot structure of sulfur trioxide, but need to draw three resonance structures, with the average of them being a representation of the molecule. Web how to draw lewis structures. Since phosphorus has 5 valence electrons, we place one dot on each side of the atomic symbol. Add up the total number of valence electrons found in the entire compound. Web drawing lewis structures for molecules with one central atom: Write the formula of each compound using the chemical symbols of each element: Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. The number of dots equals the number of valence electrons in the atom. Note, the sulfur is sp 2 hybridized giving it a trigonal planar geometry, and the unhybridized p orbital forms the pi bond with with overlap of the p orbitals of each. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Shared pairs of electrons are drawn as lines between atoms, while. Dot structures and molecular geometry. These dots are arranged to the right and left and above and below the symbol.
Lewis Structures Made Easy Examples and Tricks for Drawing Lewis Dot

3 Ways to Draw Lewis Dot Structures wikiHow

Lewis Dot Structures of Atoms and Ions YouTube
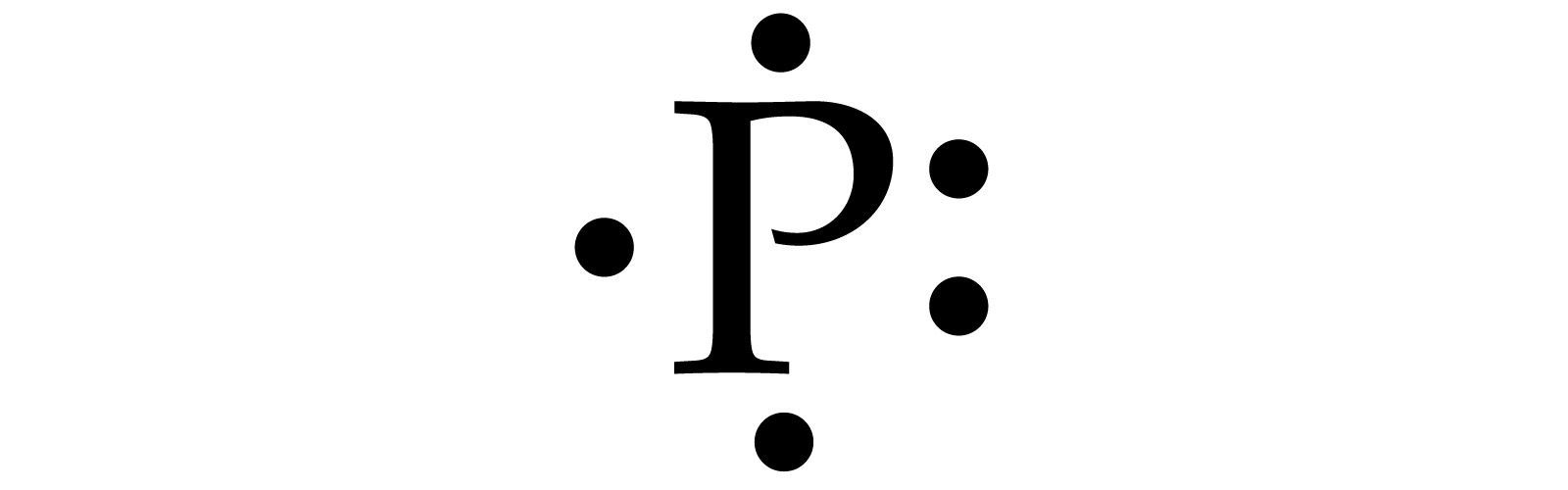
Lewis Dot Structure

How to Draw the Lewis Dot Structure for P 3 (Phosphide ion) YouTube

Lewis Structure Types

How To Draw Lewis Structures Abilitystop
Organic Chemistry How To Draw Lewis Structures YouTube

How To Draw Lewis Structures A Step By Step Tutorial

Lewis Dot Structure Definition, Examples, and Drawing
Each Dot Represents One Valence Electron.
Each Symbol Represents The Nucleus And The Core Electrons Of The Atom.
Electron Dots Are Typically Arranged In Four Pairs Located On The Four Sides Of The Atomic Symbol.
A Lewis Structure Contains Symbols For The Elements In A Molecule, Connected By Lines And Surrounded By Pairs Of Dots.
Related Post: